Could a Metal-Coating Process Help Both Manufacturers and Doctors?
August 11, 2017
Fusing metal braids at their cross-sections on catheters offers a number of potential benefits.
|
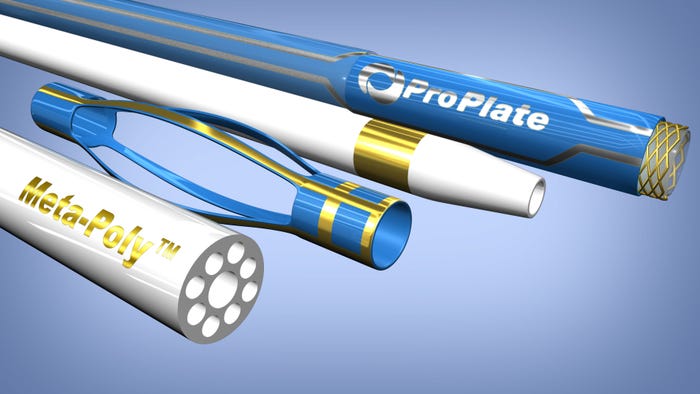
Above: Various techniques from ProPlate.
ProPlate, a provider of engineered metal coatings for medical device applications, will be highlighting its Torq-Lok metal-bonding technique for catheters at MD&M Minneapolis this fall.
The company is known for its Vizi-Band process for adding radiopaque marker bands to medical devices through gold electroplating. "Our process has allowed design engineers to place these markers where they couldn't before," Ross Peterson, marketing manager for ProPlate, told Qmed. "We've changed the idea of a marker band." For instance, through its metal-coating process, ProPlate can add numbers so that clinicians can see under fluoroscopy the exact position of each part of a device. And devices could be guided rotationally with marked symbols. The process atomically bonds these marks, eliminating the chance of any materials dislodgment during medical procedures.
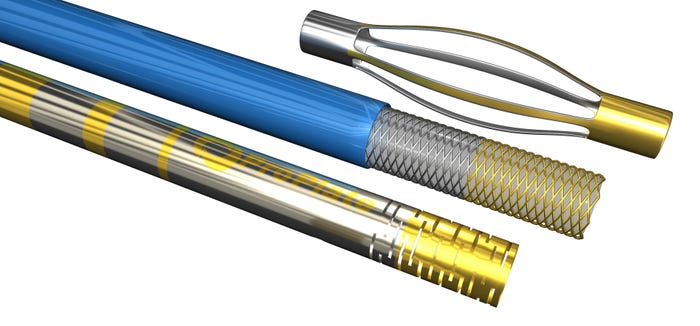
For its Torq-Lok catheter coating, the company uses electroplating to fuse together the individual cross sections of metal wire braided before it is reflowed into catheter assemblies.
|
Vizi-Band |
"Energy is lost when these sections slide over each other," Peterson explained. Through ProPlate's process, which is "comparable to microwelding each cross section," he said, "we can make it one whole structure, keeping the energy input from being absorbed by deformation of the braid and minimizing loss."
Such fusing could enable medical device designers to specify "less-complex braids, with the same torque or better, which can be a huge performance advantage in applications like microcatheters," he added. It also minimizes the chance of fraying throughout manufacturing and improves kink resistance.
The process "increases strength and can have some effect on stiffness depending on the thickness, so if you want more flexibility, we can coat a thinner layer to prevent sacrificing substantial flexibility," he said.
Some stiffness, however, can help with "pushability," which means the catheter "won't scrunch up like an accordion during medical procedures and "won't tighten up" over the interior components, he said. It also increases kink resistance without greatly impacting catheter shaft flexibility. Surgeons may have better control over such devices during medical procedures, potentially reducing procedure length as well as patient risk, the company reports.
|
Torq-Lok |
Electroplating is frequently used in other industries such as tech, energy, and automotive, but isn't as commonly thought of for its benefits in medical device applications, Peterson said. But it offers a number of new innovations for components, such as electrical conductivity on polymers, radiopacity of complex geometries, antimicrobial properties, biocompatibility (given the use of gold), joinability of dissimilar materials, and corrosion resistance, he explained.
Electroplating can create a corrosive plant/process environment, so ProPlate has invested in its process controls to offer a "proactive consistent and clean" approach, he said. For instance, the company has written its own software to proactively control its liquid electroplating chemistries. Such "software monitors the time components spent in baths as well as the temperatures of those baths," he explained. "It also controls the electrical currents that are run through parts, and we track all parts. If you keep track of these factors, you get repeatability, reliability, and traceability."
The company also provides in-house engineering of its tooling apparatuses and custom-built baths through 3-D printing, CAD modeling, and CNC milling and turning to "quickly adapt to a new idea or customers project," he said.
ProPlate is beginning the auditing process to achieve ISO 13485 registration, Peterson added. The company is also "striving toward a cleanroom appearance," he said, "but we're not the last supplier to touch the component. Our goal is to be a clean 'white-walls' operation."
In the meantime, ProPlate is working on further development of another innovation called Meta-Poly, which entails coating metal onto polymer without the use of toxic chromic acid etching. The project stems from a customer request. "The challenge is that stock polymers cannot conduct," he explains. "Water beads off, so stock polymers cannot be electroplated. But if polymer could become wettable through chemistries or other forms of etching, we can get the solutions in there to bond. It could be like Vizi-Band for some applications, but it also opens doors to create an electrical circuit on a balloon catheter. And it is not limited to catheters--customers can come to us with ideas for their projects. Electroplating polymers could help create new devices.
|
|
"We pride ourselves on helping our customers think outside the box," Peterson concluded. "They can then conceive, 'If I can combine this material with that one, I could potentially do that.'"
For more details, visit ProPlate's Web site and be sure to visit ProPlate at MD&M Minneapolis November 8-9 at Booth# 1716.
Daphne Allen is executive editor of Pharmaceutical & Medical Packaging News and a contributor to MD&DI and Qmed. Reach her at [email protected] and on Twitter at @daphneallen
About the Author(s)
You May Also Like





.png?width=300&auto=webp&quality=80&disable=upscale)
