4 Steps in Selecting Fluid Connectors for Medical Device Applications
August 7, 2014
Choosing the right fluid connectors can improve patient safety and user convenience while maximizing sealing and flow performance.
With so many risks and options for connecting tubing in medical applications, it is important to have a simple and repeatable strategy for selecting the best connector possible. The process requires a thorough analysis of the application in order to ensure connectors will be compatible with the physical, chemical, and biological environment, and be easy to use and help prevent misconnections--whether the application involves connecting air lines for a blood pressure cuff, connecting reagent supplies to a blood analyzer, or making critical connections between a patient and heart-lung machine. The connector selection process can be broken down into the decision-making steps outlined below.
|
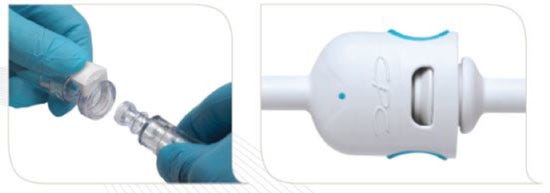
Connectors for medical applications come in a wide variety of styles and sizes. Choose styles that offer the best performance, convenience and safety. |
Step 1: Consider Safety Needs
Begin the connector selection process by considering the safety of the patients and healthcare professionals who will be using the connectors. Connectors need to be easy and intuitive to use so there is no danger of leaks, spills, or, in particular, misconnections.
In the past, the luer was a simple, universal design that provided an acceptable connection for many applications. However, today's escalation in the number of tubes and connections in medical laboratories, hospital rooms, and operating rooms has increased the danger of misconnections. Regulatory and reporting groups such as the Food and Drug Administration (FDA), the Institute for Safe Medication Practices (ISMP), and The Joint Commission (TJC) have all reported patient injuries or deaths arising from misconnections.
Due to concerns over the proliferation of luer connectors, a series of new smallbore medical connector standards is being developed by a workgroup of the International Standards Organization (ISO) and the International Electrotechnical Commission (IEC). The ISO 80369 series of standards will define noninterchangeable connectors and will impact connector selections for a range of medical device applications. As of early 2014, only Part 1 of the new standard has been released; ISO 80369-1 contains general requirements to ensure the prevention of misconnection between small-bore connectors used in different applications.
Parts 2 through 7 are in development and will provide standards for specific connector applications. ISO 80369-20 will define test methods and is expected to be released in late 2014. In the meantime, it is important to work with a company that understands the emerging standards and has developed products to answer misconnection concerns.
Step 2: Define the Functional Needs of the Application
|
Connections to medical equipment need to be secure and convenient while also preventing misconnections. |
The functional needs of a medical equipment application determine the parameters for tubing and connectors. The application also dictates whether the tubing and connectors are disposable or reusable and whether valves are required. For example, many hospitals use disposable blood pressure cuffs as one means of infection control. For the manufacturer of the blood pressure monitor and cuffs, it means selecting a connector style without a valve that is simple, low cost, and easy to secure and remove.
A blood analyzer is a more complex application, using multiple lengths of tubing and connectors to supply the machine with various reagents or cleaning chemicals. Typically, lab technicians connect bottles of reagents to the machine via tubing with connectors on both ends. Connectors with valves are designed to eliminate spills and limit air from entering the system upon disconnect, creating a closed system and a cleaner, safer work environment. Finally, the connector and tubing materials selected need to be compatible with the reagents, solvents and cleaners being used.
Consider the following factors as part of your decision-making process:
Flow requirements:The inner diameter (ID) size of the tubing generally establishes the first consideration for the size of the connector. Pressure drops across connectors and valves vary greatly by manufacturer, and some designs exhibit less turbulence and resistance to flow than others. By comparing published CV factors for various connectors (often listed in manufacturers' catalogs), design engineers can determine the pressure drop across the connector and match the correct size to their application requirements.
Media effects:Connectors and o-rings need to be compatible with the media being transferred. Start with a review of published data in chemical compatibility charts to understand what materials will work acceptably with the range of chemicals, media, or reagents in the application. Remember to consider cleaning solutions and other environmental exposure. Testing of potential product materials is always a good idea to evaluate the material's performance in the actual application. Also take into consideration the effects of gamma ray, electron beam, EtO, or autoclave sterilization on connector materials if the device will be sterilized.
Temperature and pressure: Consider the range of temperatures and pressures in the application and also consider the range of temperatures the connectors might be subjected to in storage or shipping.
Connector quality: The level of manufacturing quality of a connector dramatically affects its performance and can also play a role in aesthetics. On molded plastic couplings, parting lines (where two halves of the mold came together) and mold defects should be minimal on the connector body and absent from the first hose barb. Imperfections on barbs or threads can lead to leaks. The molded connector's gate (the point where the plastic was injected into the mold) should be free of jagged edges--poke-points that can catch or tear surgical gloves or tubing.
Fitting type: The most popular styles of hose connection are the single and multiple hose barb. Single hose barbs work well with softer tubing such as silicone rubber. However, connectors with multiple, sharp, well-made barbs, free of parting lines, provide a more secure connection over a range of tubing types.
Valve options: There are many styles of valves, and their flow rates and pressure drops vary. Connectors with integral valves prevent spills upon disconnection and prevent air from entering the system. Connectors with integral precision flush-face valves are considered true "dry-break" connectors and should be selected when there is a critical need to avoid any spills, contamination or the introduction of air upon connection.
Mounting options: In some applications, a connector is mounted to the back, side, or front panel of a machine. Metal may be preferred for strength and a higher-end appearance; however, in most applications, plastic provides a robust, cost-effective solution.
|
Manufacturers offer a wide range of fluid connectors, enabling medical product designers to choose from the best solution for their application based on safety concerns, functional needs, additional performance capabilities and materials compatibility. |
Step 3: Consider Enhanced Connector Function
In addition to connecting tubing to facilitate and control the flow of fluid, new connector technologies can bring other functionality and performance attributes to medical devices and equipment. Intelligent connectors equipped with radio frequency identification (RFID) technology allow data exchange at the point of connection. Intelligent couplings communicate by sending RFID signals between the two separated coupling halves. Data is stored on an RFID tag embedded in the passive half of the coupling, known as the insert. Looking for the tag is an RFID reader housed in the active half of the coupling, called the body. When the two coupling halves are brought within a few centimeters of each other, the reader detects the tag, reads it, and sends the tag data to the control unit running the system. The control unit can also tell the reader to write new information to the tag. Communicating couplers can transmit information that helps protect equipment, improves processes, and even saves lives in medical applications.
Another new connector approach that increases connector function is the hybrid quick disconnect, which combines the transfer of power, signal and fluids (liquids and air) in a single device. Hybrid connectors eliminate the need for multiple connections and simplify the user interface between remote tools and a device. These connectors allow technicians to quickly and safely change or replace modular tools, umbilicals, or hand pieces.
Step 4: Match Materials to the Application
The type of media flowing through a connector can influence the selection of a connector. The sidebar below lists generally available connector and O-ring materials, plus guidelines for using these materials.
Plastics
ABS: Economical, medical-grade thermoplastic that withstands gamma and E-beam sterilization.
Acetal: Strong, lightweight and economical material that has good rigidity over a broad temperature range, with toughness and durability.
Polyamide (nylon): Very resistant to wear and abrasion, with good mechanical properties at elevated temperatures.
PEEK (polyetheretherketone): An engineered thermoplastic with high-temperature, chemical and fatigue resistance.
Polycarbonate: Resistant to some chemicals, transparent and withstands sterilization for medical applications.
Polyethylene: Low-cost, chemically resistant, opaque thermoplastic.
Polypropylene: Excellent general-purpose resin that is highly resistant to attack from solvents and other chemicals.
Polysulfone: Rigid, strong and chemically resistant, it withstands repeated sterilization and higher temperatures more than other thermoplastics.
Fluoropolymers
PVDF (polyvinylidene difluoride): A tough engineered thermoplastic with a balance of physical and chemical properties that makes it suitable for high-performance applications.
Alloys
Aluminum: Lightweight metal with a high strength-to-weight ratio that is available with a durable anodized finish.
Chrome-plated brass: Rugged and attractive, this metal is excellent for high-pressure and high-temperature applications.
Die-cast zinc: Weighing about 20 percent less than brass, this durable and lightweight material withstands high pressure and high temperature.
O-ring selection
Buna-N: This is the most common O-ring material due to its solvent, oil and water resistance.
Ethylene-propylene-diene-monomer rubber (EPDM): Also known as EPR, this material offers excellent chemical resistance.
Fluorocarbon (FKM): Known for its outstanding resistance to heat, oxidation, weathering and ozone.
Silicone: Has good temperature resistance. Medical-grade silicones also meet FDA Class VI-requirements for biocompatibility in lifescience applications.
Conclusion
Although connectors are sometimes thought to be a minor component of medical equipment, they should not be relegated to an afterthought in the design process. Connectors need to be considered early in the process in order to fulfill aesthetic goals as well as product performance and patient safety objectives. Because the connector is usually the user's primary interface with the device, it plays a key role in overall perception of the design. A well-designed connector makes the medical device easy to use and enhances the overall satisfaction with the device.
Jim Brown is the business unit manager for medical markets at CPC (Colder Products Company) in St. Paul, MN. Jim has been customizing fluid connections for more than 20 years. His expertise includes applications for medical devices such as blood pressure monitoring, dialysis and surgical equipment. Jim holds a Bachelor of Science in mechanical engineering from the University of Minnesota, Institute of Technology. Jim is a member of LifeScience Alley and AAMI and currently serves as a US Expert to ISO Technical Committee 210/JWG4 developing standards to prevent misconnections of small bore-connectors.
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)
