3 Big Challenges for Medical Equipment Makers
February 3, 2014
As medical device makers face unprecedented opportunities and challenges created by a rapidly-evolving market, they need to consider the following important issues.
1. Materials Compliance and International Regulations
Device manufacturers must consider the materials and manufacturing processes that go into their products to ensure that they adhere to various regional and global material compatibility regulations. A couple key regulations include the Restriction of Hazardous Substances Directive 2002/95/EC (RoHS) and Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) standards.
|
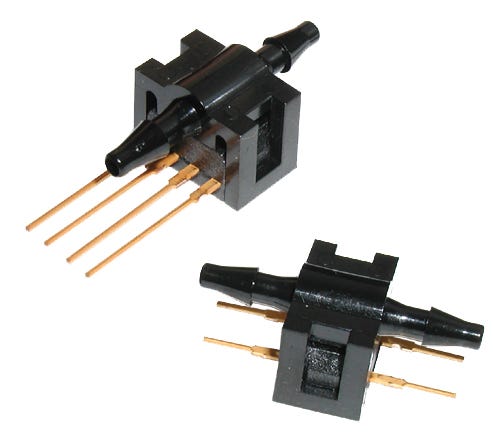
Ideal for medical applications, the Honeywell 26PC flow-through pressure sensors are designed for direct-liquid contact. |
Established by the European Union (EU) to control the levels of toxic materials in the e-waste stream, RoHS strictly restricts the use of lead, mercury, hexavalent chromium, and brominated fire retardants in electronic equipment and is rapidly becoming a globally-recognized standard. REACH addresses the production and use of chemical substances and their potential impact on both human health and the environment. This wide-reaching standard currently governs the use of over 150,000 substances within the EU and is being adopted or used as the basis for similar legislation in a number of other countries, including Australia, Canada, Japan, and Korea.
Manufacturers are required to document compliance throughout their products, supply chains, and manufacturing processes in order to obtain certification for RoHS and REACH. Device makers can dramatically reduce the time and effort to achieve certification for its products by selecting suppliers, such as Honeywell, whose products are already certified and documented as globally-compliant with key environmental regulations. Additional time and money can be saved during product upgrades by using a customized version of a previously-approved sensor design.
Learn about cutting-edge medtech technologies and trends at MD&M West, which is held February 10-13 in Anaheim, CA. |
|
Dialysis machines require highly-accurate pressure measurements of the patient's blood pressure and the dialysate fluid which clears toxins from their bloodstream. |
2. Sensors for Humid Air and Fluid Pathways
Liquid-compatible sensors are integral for devices that come in contact with humid air or fluid pathways. Sensor manufacturers now offer pressure, humidity, and temperature sensors, featuring special packaging, and designs that enable direct fluid contact with the sensor, thereby eliminating the need for work-around designs to protect the sensor. This helps simplify the design-in process and reduce part count, complexity and cost.
|
Honeywell's NBP series of basic board-mount pressure sensors are available in a wide range of shapes, sizes and I/O options. |
If medical devices involve the movement of fluids or humid gases that require measurement (such as pressure, flow, and humidity), and if the sensors are not compatible with liquid media they will require additional components to protect the sensor, which can add bulk, expense, and complexity to the device's design. But, with liquid-compatible sensors, design engineers can create compact, high-performance medical equipment while reducing design risks and streamlining development efforts.
In applications such as dialysis machines, the sensor's ability to tolerate direct contact with the dialysate solution enables the machine to deliver prompt pressure feedback to the pump controller as it is being filtered for the patient. The sensor platform is available in several application-specific configurations which integrate port options and package designs created for dialysis machine manufacturers.
3. The Need for Smaller and Portable Devices
There is a growing demand for smaller, lighter, and more portable equipment to help improve quality and cost-effectiveness in nearly every area of modern medical care. In the patient transport, intake, and ambulatory care environments smaller patient monitoring and support equipment is needed to meet space constraints and enable better and more convenient patient care. Advanced diagnostic and treatment capabilities that had been formerly reserved for operating rooms and ICUs are now offered in small, portable form factors that enable medical teams working in local clinics, field care units, and even the tight confines of medevac aircraft, to have access to this life saving technology.
Smaller respirators, infusion pumps, and vital sign monitors help hospitals improve the quality of in-room and ICU care. Compact monitors, pumps, and suction equipment provide an operating room's scrub team with easier access to their patients and the monitors, life support and treatment equipment they depend on.
The new generations of versatile, platform-oriented sensors that have recently hit the market are helping designers build smaller, more efficient medical devices. Each series of sensors is built around a sensor element which is packaged in a range of options for mechanical interfaces, mounting styles, packaging styles and I/O configurations. This allows medical device design engineers to have access to thousands of sensor packaging options to best meet their application's needs, such as making medical equipment smaller and simplifying sensor integration.
Pressure sensors, for example, provide several options for mating connections (port styles), packaging (DIP, SIP, surface mount), and outputs (analog or digital) that can be used to satisfy their applications' demanding functional, cost and board space requirements.
|
Honeywell HumidIcon digital humidity/temperature sensors are an example of how advanced packaging techniques make it practical to integrate multiple functions- temperature and humidity in this case, in a single compact package. |
The multiple benefits offered by sensors with board-mountable packages have made them one of the most popular space-saving components for medical and industrial designs. In the case of sensors which measure fluid pressure or flow, board-mount packaging enables the sensing element to be firmly attached to the device's printed circuit board (PCB) as close as possible to the patient and/or the liquid media (such as blood, chemicals or water) it's sensing.
Packaging options which combine the sensor port and a pre-integrated manifold can provide additional space savings for some applications. By eliminating the tubing and related connections between the sensor/port assembly and its target medium, can result in a simpler, more compact design.
Many medical electronic designs can also benefit from integrated sensor solutions which combine multiple functions, such as temperature and humidity, or temperature and pressure. Multi-sensor solutions can be implemented by either using sensors co-packaging with two or more sensor elements or integrating separately-packaged sensors within a compact higher-level assembly.
Robert Kim is the strategic marketing director of Honeywell Sensing and Control (Golden Valley, MN).
About the Author(s)
You May Also Like
.jpg?width=700&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)
