August 17, 2015
Along with Hospira, CareFusion has had a string of recent infusion pump recalls get Class I status from FDA.
Qmed Staff
|
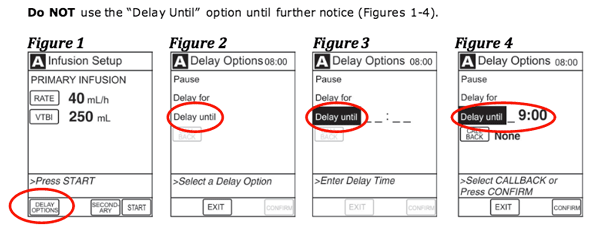
A CareFusion tip sheet warns users of the affected device to not use an Alaris pump's "Delay Until" option until further notice. |
FDA recently announced that a recall of the CareFusion Alaris syringe infusion pump has given FDA's most serious recall classification. The company has had nine Class 1 recalls of its Alaris-branded pumps in five years.
The company's other products have seen a high number of recalls as well. The company has had six Class I recalls from the beginning of 2013 to present. From 2012 to 2014, the company had 11 Class I-recalls it experienced from 2012 through 2014, six involved recalls for Alaris-model infusion pumps.
In the most recent case, the company has spotted a problem with its Alaris syringe pump module 8110 related to a channel error that triggers "an audible and visual alarm on the Alaris PC unit and a channel error on the Alaris Syringe," according to the FDA notice.
The company issued the recall based on a customer complaint related to the device's "Delay Unit" during use. In its testing, CareFusion found the same problem and also noticed that it occurred when the pump's "Multidose" feature was used but not when the "Delay For" option was selected.
The company's recall notice says that if the problem occurs and is not quickly detected by a clinician, a patient using the device could face a risk of serious injury or death.
The company recommends installing the previous version of the Alaris pump module software.
CareFusion is offering to replace the unit's syringe drive train assembly and adjust them, if needed.
The recall applies to 8110 units produced from March to September 2014.
Learn more about medical applications of cutting-edge implants at MD&M Philadelphia, October 7-8, 2015. |
Nancy Crotti is a contributor to Qmed and MPMN.
About the Author(s)
You May Also Like