The End of Renal Denervation?
January 21, 2014
Renal denervation as a high blood pressure treatment is looking less exciting by the day, with the latest bad news coming from Covidien.
The Dublin, Ireland-based medical device giant said Tuesday that it will exit its OneShot renal denervation program, which involves technology Covidien acquired through its April 2012 acquisition of Maya Medical. The move, which also includes Covidien not proceeding with its Rapid II randomized study is the result of "slower than expected development of the renal denervation market," according to a Covidien news release.
One major slowdown for development came about two weeks ago, when Medtronic announced its Symplicity system failed on efficacy in a pivotal U.S. clinical trial.
The Symplicity HTN-3 study, which involved a randomized 535 treatment-resistant hypertension patients in 87 U.S. medical centers, demonstrated that the treatment was safe. But it did not meet its goals for lowering blood pressure among those who received the treatment, versus the control group that underwent a sham procedure. Those in the control group had the option to receive the treatment after the six month assessment was over.
|
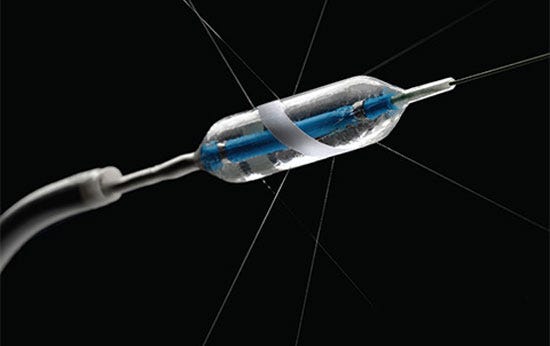
OneShot renal denervation system has a spiral electrode design that provides a standard ablation pattern. |
The news regarding the Medtronic trial led many cardiologists to criticize the hype that had surrounded the technology for years. For instance, Sanjay Kaul of Cedars-Sinai Medical Center (Los Angeles) tells MedPage Today that "[i]mplausibly large treatment effects observed in uncontrolled and unblinded studies are typically unreliable and seldom replicable in rigorously controlled randomized trials." He continues: "That is the major lesson from the Symplicity HTN-3 trial. If the data are too good to be true, they usually are!"
Covidien's move to abandon the OneShot program is but another sign of reevaluation around the technology, which has received significant media attention in recent years for its potential to benefit millions of patients with resistant hypertension. Renal denervation involves applying radiofrequency ablation through a catheter to the renal artery that supplies the kidneys with blood. The minimally invasive procedure is supposed to lower blood pressure by cutting off nerve signals to the artery.
Sanjay Kaul and other cardiologists stress that the Medtronic data does not necessarily spell the end of renal denervation. "It is too soon to abandon this procedure," Kaul notes. "We need to scrutinize the data first." He does, however, conclude: "If the treatment effect is attributable entirely to a sham effect, then it is difficult to see a future for renal denervation."
Other companies are sticking by the technology for now, even as they take stock of the situation.
St. Jude Medical, for example, called off it Enlightn IV trial in December, possibly on the assumption that Medtronic was going to beat it to the U.S. markets, according to reports in MassDevice and elsewhere. But St. Jude CEO Daniel Starks told MPMN's sister publication MD+DI that the experience with the atrial fibrillation technology, where it took many years for the technology to become widely used, indicates that, "we have a warm trail and should continue to work at it."
Boston Scientific CEO Mike Mahoney also remains optimistic about renal denervation and the company's Vessix platform, even as the company takes time to draw lessons from the Symplicity trial.
About the Author(s)
You May Also Like