Procyrion’s Milestone Streak Continues with Breakthrough Device Designation
The measure comes shortly after the Houston, TX-based company completed a $30 million Series D funding round to help with the development of its Aortix percutaneous blood pump.
July 30, 2019
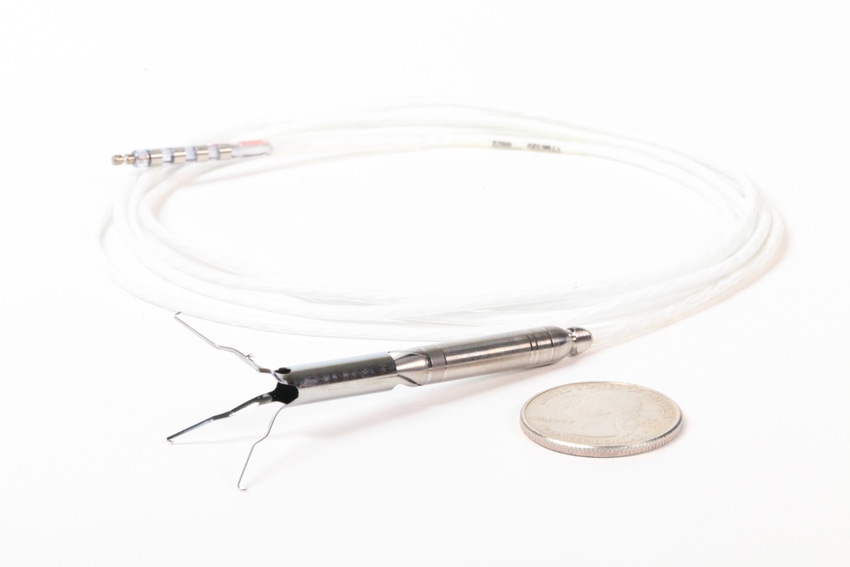
The milestones for Procyrion are coming in fast and furious. Now the Houston, TX-based company has secured Breakthrough Device designation by FDA, just a few short weeks after it closed its $30 million series D round.
Procyrion is developing the Aortix is a circulatory support device for chronic heart failure patients on medical management who have been hospitalized for acute decompensated heart failure (ADHF) with worsening renal function. The device is placed via a minimally invasive catheter-based procedure that takes less than 10 minutes.
Through this program, Procyrion can expect prioritized review of FDA submissions for the Aortix System. In addition, the Centers for Medicare and Medicaid Services (CMS) recently proposed a new rule that would provide coverage and increase payments for medical devices designated by FDA as breakthrough devices, so Medicare beneficiaries do not have to wait for access to the latest innovations.
“We believe Aortix addresses an important unmet clinical need and can fill a major gap in effective therapy options that are available for heart failure patients. This is particularly critical as this large patient population has high rates of rehospitalization and death, long lengths of stay in the hospital and high healthcare costs,” commented Eric Fain, MD, president and CEO of Procyrion. “We plan to fully leverage the benefits of FDA Breakthrough Device designation as we seek to accelerate the U.S. clinical and regulatory process with the goal of providing physicians and patients with the benefits of our novel device.”
Procyrion said the initial version of the Aortix device provides up to seven days of circulatory support for chronic heart failure patients who have been hospitalized for ADHF, have worsening renal function, and are unresponsive to medical management.
About the Author(s)
You May Also Like


