What a Difference Four Years Makes: FDA Approves Sapien Device Long after It Finds Approval in Europe
FDA recently approved the Sapien artificial heart valve from Edwards Lifesciences, which can be implanted without open-heart surgery. When inserted near the groin, the device can be threaded with a catheter into a major artery and be eventually positioned into the heart. Because it is substantially less invasive than open-heart surgery, it could provide hope to elderly patients who, in the past, would have been inoperable.
November 5, 2011
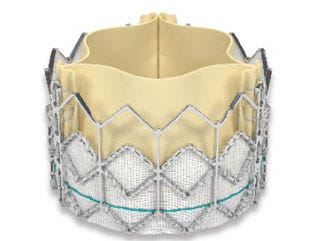 FDA recently approved the Sapien artificial heart valve from Edwards Lifesciences, which can be implanted without open-heart surgery. When inserted near the groin, the device can be threaded using a catheter into a major artery and eventually be positioned in the heart. Because it is substantially less invasive than open-heart surgery, it could provide hope to elderly patients who, in the past, would have been inoperable. Cardiovascular specialist David Holmes, MD of the Mayo Clinic called the Sapien a “game changer.”
FDA recently approved the Sapien artificial heart valve from Edwards Lifesciences, which can be implanted without open-heart surgery. When inserted near the groin, the device can be threaded using a catheter into a major artery and eventually be positioned in the heart. Because it is substantially less invasive than open-heart surgery, it could provide hope to elderly patients who, in the past, would have been inoperable. Cardiovascular specialist David Holmes, MD of the Mayo Clinic called the Sapien a “game changer.”
The regulatory approval of the device could be especially profitable for the company. According to a report from Bloomberg, sales of the valve could help double the company’s revenue in a decade. (Last year, the firm had sales of $1.5 billion.)
Device Approved Four Years Earlier in Europe
Somewhat buried in the New York Times article on the device's introduction was a line that the Sapien “has already been approved for four years in 40 countries, including most of Europe.”
Incidentally, the regulatory nod marks the first time that FDA has approved such a transcatheter device. In Europe, however, the second-generation version of the Sapien device, the Sapien XT received the CE Mark in 2010. Medtronic also has a transcatheter device, the CoreValve, on the market in Europe.
The slow approval trend reflects a broader trend. |
The slow approval of Sapien in the United States reflects a broader trend. Class III devices, such as heart valves and other life-sustaining implantable products are approved by the PMA regulatory path. From 2003–2007, that path took an average of 15.5 months, according to analysis from Ernst & Young. In 2010, it took nearly double that—27.1 months. A report titled “FDA Impact on U.S. Medical Technology Innovation” compared PMA and the European CE Mark numbers in 2009. For U.S. companies clearing products in Europe, it took an average of 11 months from first communication to certificate. U.S. companies had to wait 54 months, on average, from the first communication to final approval. In any case, it’s hard not to look at data like this and not think that something is amiss at the FDA. Especially considering that the safety rates and adverse rate numbers for devices in the United States and Europe are almost identical.
There should be a greater sense of urgency at the FDA to address the reasons that cause a large number of U.S. companies to go offshore for regulatory approval. Sure, FDA is the only game in town in the United States. And this country represents a huge market. But the potential for the U.S. device industry is threatened by FDA's cumbersome policies. Of course, protecting public health is absolutely necessary. But when growing numbers of patients are going abroad as medical tourists, and when other countries are able to train doctors to use more advanced technology than is available here because the technology is acutally available in those countries, well, that’s a problem. As Thomas Fogarty, MD, told me in a recent phone interview, “FDA has got to do something now. Now.” Any solution that looks for improvement one year from now, two years from now, three years from now, isn’t going to cut it.
Update: FDA recently approved trial expansion for the next-generation Sapien XT device to include lower-risk patients.
Related Content
You May Also Like
.png?width=300&auto=webp&quality=80&disable=upscale)

