Medtronic Warns That Cardioversion May Damage Neurostimulation Devices
Cardioversion in patients implanted with the Vanta neurostimulator may damage the electronics of the device, Medtronic warns.
June 12, 2023
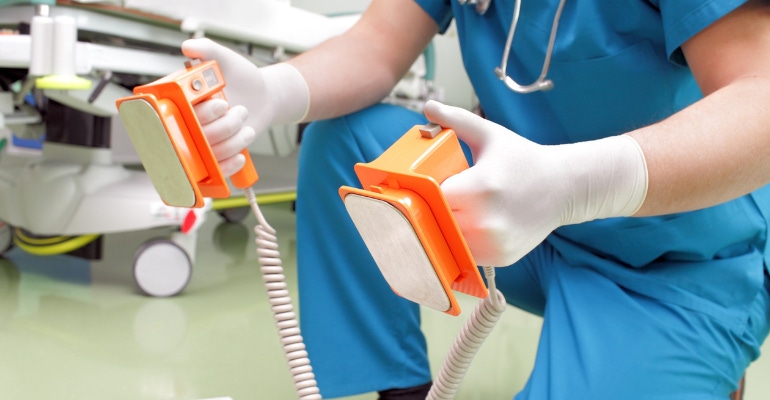
Medtronic alerted healthcare professionals in Europe to the risk of cardioversion-related damage to the Vanta implantable neurostimulator (INS), model 977006,
During cardioversion, healthcare workers use a defibrillator to shock a patient's heart back into a normal rhythm. It's typically used for atrial fibrillation and atrial flutter, but it also helps with other fast or irregular heart rhythms. While this can save your life if you’re having a ventricular arrhythmia, Medtronic said the procedure may damage the electronics in the Vanta INS device, making it unresponsive and non-functional.
As of April 19, Medtronic had received two complaints concerning this issue from patients implanted with a Vanta INS, both of which have resulted in the implantable device having to be removed, the company noted in a June letter to healthcare professionals.
Medtronic said it has identified programming mitigations that reduce the risk for damage. In the event of damage to the device, surgical replacement of the INS is required to restore stimulation therapy. The potential for electrical damage to the Vanta INS can be minimized by temporarily reprogramming the patient’s Vanta INS for cardioversion procedures as recommended in the device manual.
Medtronic updated the manual and applicable labeling to add specific recommended neurostimulator settings and programming considerations for cardioversion procedures. The company said it also updated the patient therapy guide so patients will know to tell their doctor about any upcoming cardioversion procedures so that the Vanta INS can be properly programmed. Both Vanta INS guides are online.
Required actions for healthcare professionals managing patients implanted with Vanta INS devices undergoing cardioversion
Communicate the updates to patients regarding programming considerations for cardioversion procedures with patients implanted with Vanta INS devices.
If a patient will be undergoing a cardioversion procedure (e.g., nonemergent scenario) refer to the recommended neurostimulator settings and programming considerations in the manual which include not turning off your patient’s Vanta INS device and reprogramming the settings.
After a cardioversion procedure, confirm that the neurostimulator is functioning and programmed as intended.
For patients who are considering a new or replacement implant, and who have concomitant conditions such that they may require future cardioversion, talk to them about the relative benefits and risks associated with proceeding with a Vanta INS device.
Share the urgent field notice with all those who need to be aware of this issue within your organization or to any organization where the potentially affected devices have been transferred and maintain a copy of the notice in your records.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
