Lessons from the Evolution of the Automated External Defibrillator
Researchers credit rapid prototyping and user-centric design key to developing the small, light, and portable automated external defibrillator.
March 30, 2022
Not too many years ago, the chances of surviving a sudden cardiac event outside of a hospital setting were slim. Realizing the need for a life-saving treatment that could be used almost anywhere, and by almost anyone, the Royal Victoria Hospital-Belfast, under the direction of Dr. Frank Pantridge and Dr. Geddes, launched the world’s first mobile coronary care unit, which included the world’s first portable defibrillator.
“Pantridge’s vision in the 1950s was that a defibrillator should be beside every fire extinguisher,” said Professor Dewar Finlay, Interim Associate Dean, School of Engineering, Ulster University.
Although they had developed a mobile cardiac care unit, it was not convenient to use, as it weighed 110 lb. This was mainly due to the lack of miniaturized electronic components, particularly those required to power a defibrillator and provide the electrical energy needed to produce the required shock.
The research team, which formed the company, HeartSine in 1988, now owned by Stryker, continued to refine the device to make it more lightweight and portable. The Ulster University academics have since gone from a team of five employees to being acquired by one of the world’s largest medtech companies by a process of constantly refining and improving their devices.
“Much of our early work was focused on the development of suitable technology to allow this miniaturization to be realized,” Finlay explained. “These developments, including the integration and in-house research of new flat-panel displays, compact capacitors, flexible defibrillator pads, high-density batteries, and embedded software, primarily led to a device that was suitable for transportation in an ambulance to a patient suspected of having a cardiac arrest.”
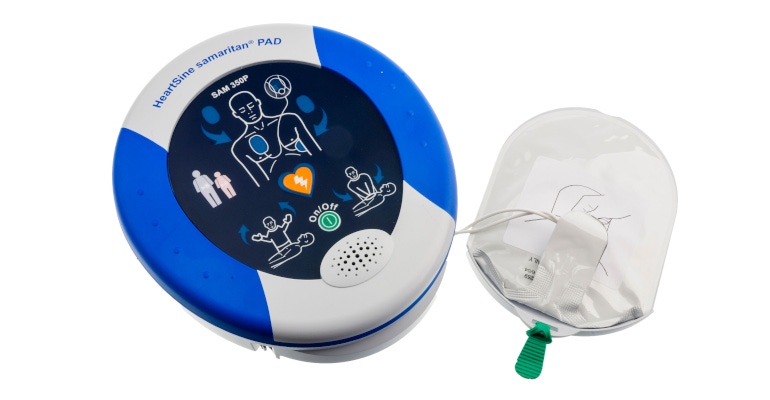
This work provided the platform for which the modern wall-mounted automated external defibrillator (AED) is based on, Finlay said. HeartSine’s flagship product, the HeartSine Samaritan public access defibrillator (PAD; pictured above), has been deployed in thousands of facilities in more than 70 companies and in more than 30 languages.
The evolution of the device was based on many years of experience, explained Professor James McLaughlin, Head of School of Engineering, Ulster University. “As with all of Ulster University’s work in medical devices and related technology development, we have learned that many iterations of the technology are required to facilitate a final viable solution,” he explained.
One key example of this, McLaughlin stated, has been the establishment of its Rapid Prototyping Laboratory in the past decade. “This concept, as the name suggests, allows for rapid prototyping of device components and subsequently expedites the process of getting device prototypes into the end users hands,” he said.
“Also, after many years we have now adopted much improved user-centric design, whereby clinicians and patients are part of the design process,” he continued. “This allows early stage feedback to be incorporated into prototypes for further evaluation within Living Lab settings.”
Finlay said the relationship with Stryker continues, from the spin out of HeartSine to the present-day operation of the company in Belfast. “The current relationship is realized through a number of funded research projects and a number of joint PhD programs,” Finlay noted. “These projects/programs continue to lead the way on research ranging from the development of advanced biosignal processing algorithms through to the development and refinement of UX (user experience) aspects of medical devices,” he said, noting that Ulster University’s healthcare technology research continues on a number of broader areas that include development of AI algorithms for diagnostic applications in cardiology and point-of-care testing technology that has been particularly relevant during the pandemic.
McLaughlin said that, moving forward, his team is in the process of establishing the University’s £40 million Centre for Digital Healthcare Technologies that will be integral to the Belfast Regional City Deal. “We see this Centre as providing the next generation of medical device development platform by facilitating improved stakeholder engagement through the Living Lab concept,” he said.
About the Author(s)
You May Also Like




