Latest Boston Sci Deal Shakes up Mitral Repair Market
The Marlborough, MA-based company strengthens its structural heart portfolio and gains a deeper foothold in a market where Medtronic, Abbott Laboratories, and Edwards Lifesciences have already made significant acquisitions.
December 27, 2018
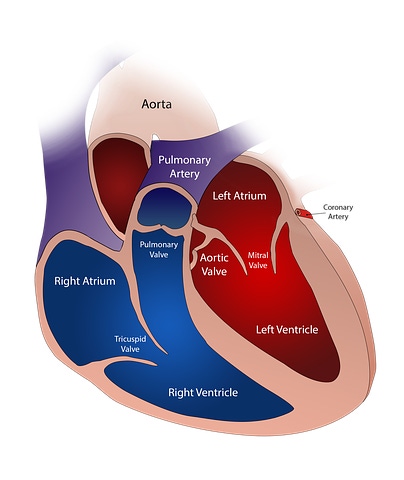
Boston Scientific is slipping in one more deal (maybe?) before 2018 ends, and it could have significant implications. This time out, the Marlborough, MA-based company will gain a stronger foothold in the transcatheter mitral repair and replacement market by exercising its option to acquire the remaining shares of Millipede Inc.
The deal will have Boston Scientific doling out $325 million with a $125 million payment becoming available upon achievement of a commercial milestone. The Millipede acquisition is set to close prior to the end of 1Q19.
Boston Scientific’s latest acquisition target is developing the Iris Transcatheter Annuloplasty Ring System for the treatment of patients with severe mitral regurgitation (MR), who are not able to tolerate open-heart surgery.
In an email sent to MD+DI, Boston Scientific said it believes “both transcatheter mitral repair and replacement are high-growth segments, as there is a large and currently underserved patient population with severe MR that has compromised heart function and is thereby unable to tolerate open-heart surgery to repair or replace the leaking valve.”
The company went on to say that, “transcatheter mitral remains a significant potential market opportunity, and there’s ample runway for it to position itself as a category leader in this space, particularly with the features of the IRIS device which are designed to have applicability across a wide range of patient populations.”
Mike Matson, an analyst with Needham and Co. LLC, wrote in research notes that the IRIS transcatheter annuloplasty ring system can accommodate both tricuspid and mitral valve repair, which can open up opportunities for Boston Scientific.
“Given Millipede’s clinical focus on mitral valve repair, we expect Boston Scientific to focus more on the mitral application but see potential TAM upside with tricuspid optionality,” Matson wrote in research notes.
The IRIS system uses a complete annuloplasty ring to reduce the size of a dilated mitral annulus. Millipede’s Iris is delivered via a transcatheter-transseptal delivery system, and can be used as a stand-alone device, or in combination with other technologies in patients with severe MR.
"Upon commercialization, we believe the IRIS system can meet the needs of a currently underserved patient population that requires physiological, less invasive options to treat functional mitral regurgitation in patients with progressive heart failure," Ian Meredith, AM, executive vice president and global chief medical officer, Boston Scientific, said in a release. "This device is designed to be highly customizable to a specific patient's mitral anatomy and disease state, and is repositionable and retrievable to promote a high-quality outcome."
The deal shouldn’t be surprising because in January of this year, MD+DI reported Boston Scientific had taken a $90 million stake in Millipede and could acquire the Santa Rosa, CA-based company within the next year or two.
This isn’t the first mitral repair company Boston Scientific has invested in. Back in December of 2016, Boston Scientific made some key investments into Neovasc. However. Matson said that Millipede is a much better deal for Boston Scientific than Neovasc at this point. Neovasc has struggled to make earnings expectations this year and saw its shares drop after it missed the mark in 3Q18.
“While there are still clinical, commercialization, and integration risks and Boston Scientific is paying a high price for Millipede, we believe this transaction could lay the foundation for the mitral leg of Boston Scientific’s Structural Heart franchise, particularly given Neovasc’s issues,” Matson wrote in a research note.
Is the Millipede Acquisition Right on Time?
Boston Scientific, which was named Editor’s Choice for Medtech Company of the Year in part because of its significant M&A activity (10 deals in 2018!), said the transcatheter mitral valve and repair market is estimated to reach about $1 billion by 2021.
But is Boston Scientific too late to hop on the transcatheter mitral repair and replacement party, or is the company right on time? In 2015, many of Boston Scientific’s larger competitors began buying up smaller mitral valve repair companies.
Edwards Lifesciences, a pioneer in the transcatheter valve replacement (TAVR) market, set the tone for the 2015 mitral valve buying spree, by spending up to $400 million to acquire CardiAQ Valve Technologies and its transcatheter mitral valve replacement (TMVR) system.
In 2016, Edwards would go on to acquire Yehuda, Israel-based Valtech Cardio Ltd. for about $340 million, with about $350 million in milestone payments.
Shortly after Irvine, CA-based Edwards made its intentions clear for the market in 2015, Abbott Laboratories announced it would acquire Tendyne, a maker of a TMVR system, for about $250 million. The Abbott Park, IL-based company followed up with an unspecified investment in Cephea Valve Technologies, with an option to buy the firm down the road.
Medtronic followed suit in the 2015-buying-spree by putting up $458 million for Redwood, CA-based Twelve Inc.
Key Trial Developments
Boston Scientific will have to contend with many of the players in the space moving into key parts of their clinical trials.
Most recently LivaNova, a company that has been in the process of reinventing itself since 2017 concluded its PRELUDE feasibility study of its Caisson TMVR system. The London-based company said in August that it would turn its efforts toward enrolling patients into its INTERLUDE CE mark trial and finalize the protocol for, ENSEMBLE, the U.S. pivotal trial.
During this year’s Transcatheter Cardiovascular Therapeutics (TCT) conference, Or Yehuda, Israel-based Cardiovalve announced it would launch the AHEAD US Multicenter Study. The company also presented details of the first-in-human procedure with the transfemoral mitral valve system during TCT.
In July of 2018, Abbott initiated SUMMIT, a 1,010 pivotal study of the Tendyne TMVR system. In an email, sent to MD+DI shortly after the SUMMIT announcement was made, Abbott said enrollment in the study was anticipated to be completed within four years and the primary endpoint would be evaluated at one year, with total patient follow-up being in five years. The firm said regulatory submission is expected one year following the trial conclusion.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
