JenaValve Wins IDE Approval for TAVR Trial
The Irvine, CA-based company said its ALIGN-AR pivotal trial will evaluate its Trilogy Heart Valve system.
August 16, 2021
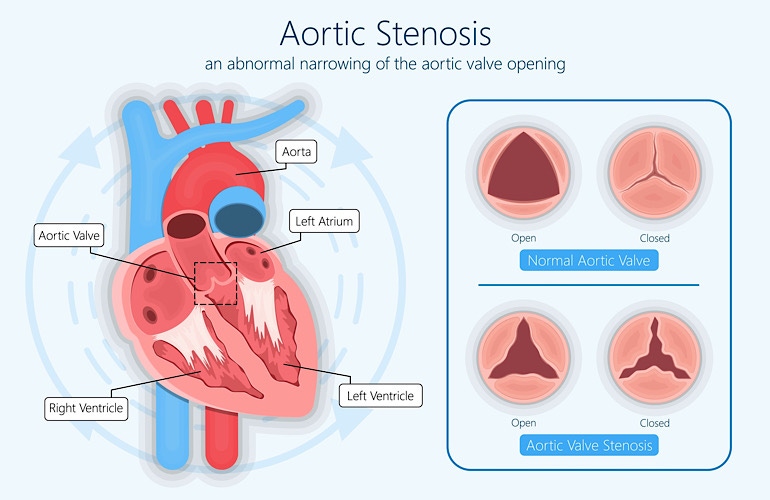
JenaValve has won IDE approval for a pivotal trial of its transcatheter aortic valve replacement system. The Irvine, CA-based company said the ALIGN-AR pivotal trial will evaluate its Trilogy Heart Valve system.
ALIGN-AR will look at how the valve treats severe symptomatic, high-surgical risk aortic regurgitation.
“We are pleased the FDA granted this IDE approval allowing us to continue our pursuit of an aortic regurgitation indication for the Trilogy Heart Valve System,” said John Kilcoyne, JenaValve’s CEO. “We believe the Trilogy Heart Valve System’s unique design provides patients with a minimally invasive transfemoral procedure that has the potential to change the treatment paradigm for what I believe is a vastly underdiagnosed and undertreated AR patient population.”
The company had a busy year in 2020. JenaValve won breakthrough device designation for the Pericardial TAVR system. Shortly after, the company raised $50 million in equity financing.
If the firm can obtain approval for its valves, then JenaValve could fill in a gap left when Boston Scientific stopped investing in the Lotus Edge platform.
Marlborough, MA-based Boston Scientific retired the entire line citing complexities associated with the product delivery system. Boston Scientific shifted focus to its Acurate neo2 TAVR, its Sentinel embolic protection device, and other high-growth areas of its portfolio. It should be noted, however, that the Acurate neo2 TAVR system isn't expected to reach the U.S. market until 2024.
About the Author(s)
You May Also Like




