FDA Grants Breakthrough Designation for Heart Failure Device
Portland, OR-based VisCardia is developing VisOne, an implantable system that delivers synchronized diaphragmatic stimulation (SDS) therapy for improving cardiac function.
April 23, 2020
VisCardia has been granted Breakthrough Device Designation for an implantable technology to treat heart failure (HF).
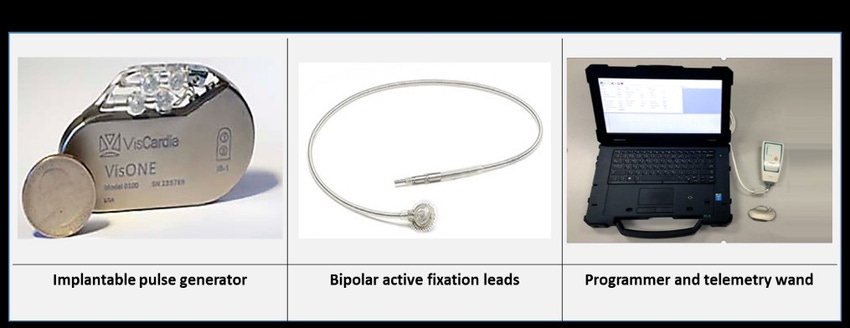
More specifically, the Portland, OR-based company’s VisOne is a device that treats moderate to severe HF with reduced ejection fraction and preserved ventricular synchrony. The system delivers synchronized diaphragmatic stimulation (SDS) therapy.
Gregg Harris, VP of Clinical and Regulatory Affairs spoke with MD+DI about VisOne and how the device has the potential to make a difference in the lives of HF patients.
“Other technologies are usually focused on strengthening the heart which causes the heart to use more oxygen and those kinds of things,” Harris told MD+DI. “Our technology makes it easier for the weak heart to pump.”
Harris added, “We implant two bi-polar leads on the underside of the diaphragm using a laparoscope. It’s minimally invasive and it takes two small half-inch incisions that allow us to plant the leads onto the diaphragm. We place a small generator subcutaneously in the abdomen, which detects the cardiac activity. It then sends a small shock to the diaphragm. This just stimulates a small portion of the diaphragm. It doesn’t affect breathing at all.”
The company said when stimulating the diaphragm below the heart synchronously with the cardiac cycle, the device has the potential to be able to modify the pressures within the chest cavity. VisCardia noted the device also has the potential to lower the pressure to allow better filling of the heart and then gives a rebound to allow better emptying of the heart as well.
“What we end up doing is improving the amount of blood flow through the heart without impacting the contractility of the heart,” Harris said.
VisOne hasn’t been approved by FDA, but the breakthrough device designation can help the company push forward with its efforts to eventually get the technology on the market.
“[The breakthrough device designation] opens up a lot of doors for us to collaborate with [FDA] to get a good study designed,” Harris said.
Last month, the company completed the VisOne Heart Failure pilot study in Europe. The primary measures of the study were safety of the minimally invasive, surgical implant procedure and implanted system while collecting observational data on the patients' HF status, including acute hemodynamic parameters, cardiac function, exercise tolerance, and Quality-of-Life.
In a March release discussing the pilot study, Prof. Dr. Michel Zuber, University Hospital in Zurich, Switzerland said, “Our objectives were to demonstrate that the VisONE implantable therapy can be delivered on a safe and chronic basis, and the improvements to clinically significant endpoints corroborate those observed during our early proof-of-concept studies. Those objectives were met, and the investigators will share the promising results through publications in peer-reviewed journals and conferences in the coming months.”
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
