Brazil’s Growing Economy Fuels Vascular Delivery Device Sales Despite Market Limiters
The vascular delivery (vascular access) device market in Brazil is valued at nearly $59 million and growing. Will high import prices and a bifurcated public/private system impede the market?
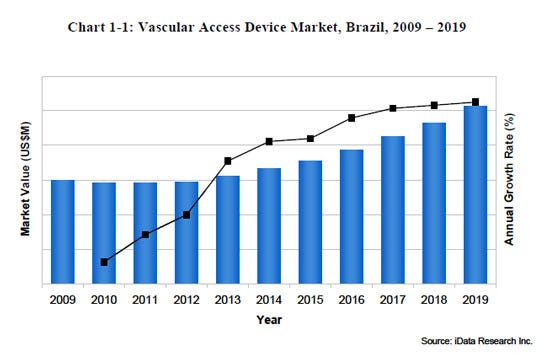
The vascular delivery (vascular access) device market in Brazil is growing and was valued at nearly $59 million in 2012. Recent growth over is primarily due to the increasing size and age of the Brazilian population, but is also because of the increased access to medical devices and procedures as their overall economy strengthens. Vascular access devices are used for gaining access to a patient’s bloodstream via the veins. They can deliver a wide range of treatments including fluid infusion, blood draws, parenteral nutrition, antibiotics, chemotherapy and dialysis. The vascular access device market in Brazil includes peripherally inserted central catheters (PICCs), central venous catheters (CVCs), implantable ports, port or Huber needles, dialysis catheters, and syringes and needles. The PICC, dialysis catheter, and total conventional implantable port market are the fastest-growing, however overall growth is being influenced by imposing government policies and import tariffs.
Demographic Demands
Driven by the increased access to healthcare provided to low- and middle-income families in Brazil, the vascular access device market is estimated to reach a value of over $100 million by 2019. The ability to infuse medications or fluids into a patient’s bloodstream is indispensable to modern medicine. In 2012, the majority of patients who were hospitalized in Brazil received some sort of vascular access treatment during their stay. The rise in demand for vascular access devices in Brazil is proportional to the number of people who require medical care each year. As of 2011, the Brazilian population was growing at approximately 0.9% per year, with the distribution shifting toward an older population due to declining mortality rates in the older demographic and reduced fertility rates in younger age groups. Approximately 6.7% of the total Brazilian population is aged 65 or older. Moreover, the prevalence of obesity in Brazil has been on the rise since 2006 with nearly half of the total population considered overweight. As the population ages and rates of obesity rise, the number of individuals requiring medical care will also grow, driving overall growth in the Brazilian vascular access market.
Another significant market driver is medical tourism, which is becoming a larger portion of many healthcare markets around the globe. Brazil has become one of the major destinations for medical tourism with the key therapeutic areas including oncology, cardiology, orthopedics, and neurology. Some of the factors attributed to this increased growth in medical tourism include the modernization of hospitals and the improvement to Brazil’s infrastructure in the form of roads, hotels, and airports. By 2015, it is expected that medical tourism will contribute approximately $2 billion to the Brazilian economy.
The Growth of Vascular Delivery Device Segments: PICCs, Dialysis Catheters, Implantable Ports
The leading and fastest-growing segment is PICCs, which have a wide range of applications and are commonly used for delivering chemotherapy and antibiotics, as well as drawing blood. This market is underpenetrated in Brazil. Growth is driven by double digit increases in unit sales as PICCs begin to cannibalize unit sales from competing device markets such as CVCs and peripheral intravenous catheters (PIVC). However, average selling price (ASP) decreases are also being seen in all of these markets, which is causing the market values to show limited growth or in some cases, a decline. In 2012, the total PICC market in Brazil was valued at $27.4 million, increasing from the previous year. Unit sales have been progressively increasing since 2007 and are expected to continue to increase until 2014. After 2014, unit sales will still continue to increase, but the year over year growth will start to equalize and stabilize. Antimicrobial and power-injectable PICCs are becoming more common in more established PICC markets such as the U.S. and Europe; but in Brazil they have very little market penetration. These devices tend to sell for a higher price than conventional devices. Their increased presence in the Brazilian market has the opportunity to increase average selling prices.
The second largest market in terms of market value is the dialysis catheter market. This market is composed of acute, conventional chronic, antimicrobial chronic, and peritoneal dialysis catheter segments. Very minimal market penetration has been seen for antimicrobial chronic catheters in some select regions of Brazil. Prices for acute dialysis catheters are declining, as these devices become more of a commodity product; there is little differentiation or new products in this segment. The use of chronic hemodialysis catheters is increasing in Brazil, especially in the low-income and middle-income sectors of the population, which, through recent changes and programs instigated by the federal government, now has more access to many healthcare services, such as dialysis. Compared with the hemodialysis markets, the peritoneal dialysis catheter segment has always been a small market in terms of unit sales and market value.
Another strong segment in the Brazilian vascular device market is the total conventional implantable port market used for oncology. In 2012, approximately half of the oncology patients in Brazil were treated through the public system while the remaining 50% were treated through private insurance. The Brazilian market is a split between the public and private. The Unified Health System (SUS) in Brazil is very strong in the oncology market, where products like implantable ports are commonly used.
The total market in Brazil is divided between manufacturers’ whose products are reimbursed by National Insurance, Sistema Unico de Saude (SUS) and those insured privately. Thus the market is split between public and private healthcare systems. Both of the public and private markets receive financial assistance from the government. The Brazilian government has been steadily increasing the amount of money devoted to the healthcare sector, but these increased expenditures have not kept up with demand, limiting the overall market growth. There is a wide price gap between the two sectors where average selling prices in the SUS system are much lower than prices in the private sector. Prices are stable in the public market since they are standardized so that all competitors are confined to the same price. However, ASPs have been moderately declining in the private market as competitors undercut one another. Thus the overall ASPs will continue to decline at a moderate rate over the next few years.
The Brazilian government has also expanded mandatory coverage policies while imposing limitations on how much the price of existing policies can be increased. These actions have caused service providers to raise the price of new policies in an attempt to recoup costs. Due to the increased price of these new policies, it is expected that people will migrate from private plans to public plans. This migration places financial stress on the market and will limit future growth.
Another market limiter medical device manufactures face is import tariffs. For a medical device to be imported into and distributed in Brazil, the device must be registered with the Brazilian National Health Surveillance Agency (ANVISA). This registration process can be lengthy. In 2012, the average length of time from filing to approval was three months to two years. Those products coming from other countries that are also part of MERCOSUL, the Latin American free trade agreement, have a more streamlined registration process and are approved more quickly. Once a company’s device is approved by ANVISA, it can still be subject to high import taxes and tariffs, causing it to sell for a considerably higher price than if the device were produced locally. Companies can bypass these import taxes and tariffs by producing the majority of the device components in Brazil. Even so, these policies have been a limiting factor in the Brazilian market.
Despite market limitations, Teleflex/Arrow is succeeding in the Brazilian vascular access device market with nearly a quarter of the total market share. Teleflex was the leader in the PICC market with a majority market share. Prior to its acquisition of Arrow International in 2007, Teleflex was not involved in vascular access. Thus, Teleflex’s vascular access market share is based entirely on Arrow’s product lines. The ARROWg+ard coating technology was the first of its kind in the CVC market and has also proven to be very effective with its PICCs. Teleflex markets and distributes its diverse product portfolio in over 130 countries.
Other competitors present in the Brazilian market for vascular access devices include, but are not limited to: C. R. Bard, Becton Dickinson, Medcomp, Joline, B. Braun Covidien, KFF S.A., Smiths Medical, Vygon, Baxter International and Fresenius Medical Care.
Conclusion
As Brazil’s economy strengthens and its population grows with an increasing need for vascular delivery devices, the PICC, dialysis catheter, and total conventional implantable port market segments in particular will expand. The vascular access device market in Brazil is relatively new and the penetration of vascular access devices is quite low. Market leaders such as Teleflex/Arrow, C. R. Bard, and Becton Dickinson are currently leading Brazil’s underpenetrated medical device markets despite the Brazilian government’s mandatory coverage policies and price limitations as well as ANVISA product registration and approval requirements. Overall, vascular delivery device sales are expected to flourish as they meet the needs of a growing population of both local citizens and tourists, fostering a thriving multimillion dollar market.
Additional Information
The information contained in this article is taken from one of a series of detailed and comprehensive global reports published by iData Research covering Brazil, U.S., Australia, Japan, South Korea and 15 countries in Europe. For more information and a free report synopsis, please contact iData Research at [email protected]
Kamran Zamanian is president, CEO, and a founding partner of iData Research Inc. (Vancouver, BC). He holds a bachelor’s degree in engineering from the University of Dundee and earned master’s and doctorate degrees in market research and technology from the University of Manchester. Reach him at [email protected].
Jamie Stilborn is head of research and product development for iData’s global series on the interventional cardiology, peripheral vascular, vascular access, cardiac rhythm management, cardiac surgery, and infusion therapy markets. His background in project management for Biotech and Environmental Science companies and keen interest and attention to detail enhances his many specialties and contributions at iData. These include data analysis and presentation, scientific and technical writing, team management, computer science, and statistical analysis. He can be reached at [email protected]
Related Content
Homegrown Orthopedic Joint Makers Gain Marketshare in Asia
Market Drivers: U.S. Orthopaedic Bone Graft Substitute Market
Cardiology: China’s Impact on Market Demand
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
