Bos Sci's Lotus Heart Valve is Doing Well in Europe
Boston Scientific may be late to the TAVR space, with competitors like Medtronic and Edwards Lifesciences firmly entrenched, but in Europe doctors seem to like Boston's Lotus device.
July 27, 2015
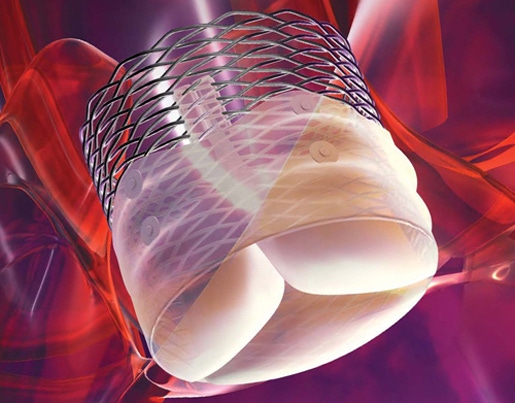
Boston Scientific's Lotus TAVR Valve System |
Boston Scientific's second-quarter performance in its cardiac rhythm management division may have been underwhelming, but some of its novel products are finding good, early adoption.
One of those is its Lotus transcatheter aortic valve system that replaces the diseased heart valves of patients with aortic stenosis. The product, which is approved in Europe, appears to be gaining traction in a pretty crowded field of competitors across the pond.
"We have about a 90% reorder rate with our existing customers in TAVR which is great given the number of competitors in Europe," said Michael Mahoney, Boston Scientific's CEO, told analysts in the second-quarter earnings call. according to a transcript from Seeking Alpha. "Once customers use Lotus, 90% of the time they continue to reorder consistently. And we have about what we estimate a third of the market share in the accounts that we're currently penetrated in Europe."
Here are just a few companies with approved TAVR products in Europe — Edwards Lifesciences, Medtronic, St. Jude Medical, and Direct Flow Medical.
Boston Scientific's early good showing in Europe could be a positive sign of how the company might fare, once the product is approved for sale in the U.S.
Previously some analysts speculated that the Marlborough, Massachusetts company might be too late to the TAVR market as Medtronic and Edwards Lifesciences dominate the field. By the time the product wins approval in the U.S., scores of physicians will already have been trained in the products made by competitors.
But Boston Scientific executives, in the past, have expressed confidence about the clinical quality that the device has. For instance the Lotus valve has shown to have the lowest paravalvular leak, a major complication of the TAVR procedure, among all competing devices. Keith Dawkins, the company's executive vice president and chief medical officer, has commented earlier that the device is easy to use and gives the physician full control.
In the U.S., Boston Scientific is enrolling patients in the Reprise III that is studying its Lotus valve against Medtronic's CoreValve device. The study is expected to complete enrolling patients — totaling more than 1,000 — by the end of this year.
Arundhati Parmar is senior editor at MD+DI. Reach her at [email protected] and on Twitter @aparmarbb
To learn more about medical devices and trends in the marketplace, attend the two-day MEDevice San Diego conference, September 1-2 |
You May Also Like


