Bioabsorbable Heart Valves Tried Out in Children
October 3, 2016
The Swiss-Dutch medical device company Xeltis has launched a multi-centered feasibility trial.
Chris Newmarker
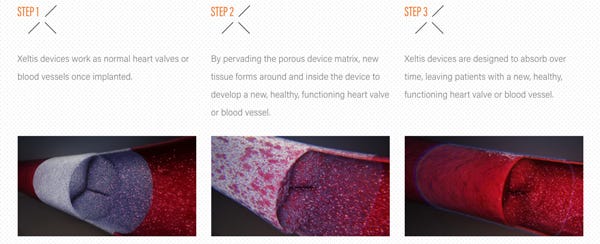
Three children so far have been surgically implanted with Xeltis's bioabsorbable pulmonary heart valve as the Dutch and Swiss medical device company kicks off its "Xplore-I" clinical study.
The first operations took place at the Gottsegen György Hungarian Institute of Cardiology's Pediatric Cardiac Centre in Budapest, Hungary, and University Children's Hospital in Krakow, Poland.
The first patients have been discharged from the hospital, with the implants performing according to expectation, said Zsolt Prodan, MD, head of congenital heart surgery at Paediatric Cardiac Centre in Budapest. Prodan performed the first two interventions in July.
Xeltis's cardiovascular implants are made of bioabsorbable polymers. The porous nature of the implant encourages the growth of new, healthy tissue to create a new heart valve as the bioabsorbable valve melts away. Xeltis boasts that the device is the "first-ever cardiovascular regenerative medicine therapy based purely on a bioabsorbable medical device implant."
"Reconstruction and replacement of diseased heart valves in children using patients' own tissue could help reduce the risk of complications and of re-interventions observed with animal and human donor implants," said Thierry Carrel, MD, principal investigator of the Xplore-I study, and professor of surgery at the Clinic for Cardiovascular Surgery at University Hospital Bern (Switzerland).
"We are quite confident regarding this technology, since children from the precursor feasibility study on bioabsorbable blood vessels demonstrate excellent results over two years after implantation," Carrel added.
The Xplore-I study will track survival rate of its participants, 2 to 21 in age, undergoing right ventricular outflow tract reconstruction, a procedure normally performed on children with congenital heart defects.
The Xeltis pulmonary valve presently has a humanitarian use designation from FDA. The company has closed on $33 million in series B financing.
Chris Newmarker is senior editor of Qmed. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
[Image courtesy of Xeltis]
About the Author(s)
You May Also Like
.png?width=300&auto=webp&quality=80&disable=upscale)

