Renal Denervation for hypertension was all the rage a few years ago, but after a failed clinical trial from Medtronic, activity in the space evaporated. Now, Ablative Solutions is one of several companies helping to reignite the renal denervation market with two large studies of its technology.
July 24, 2019
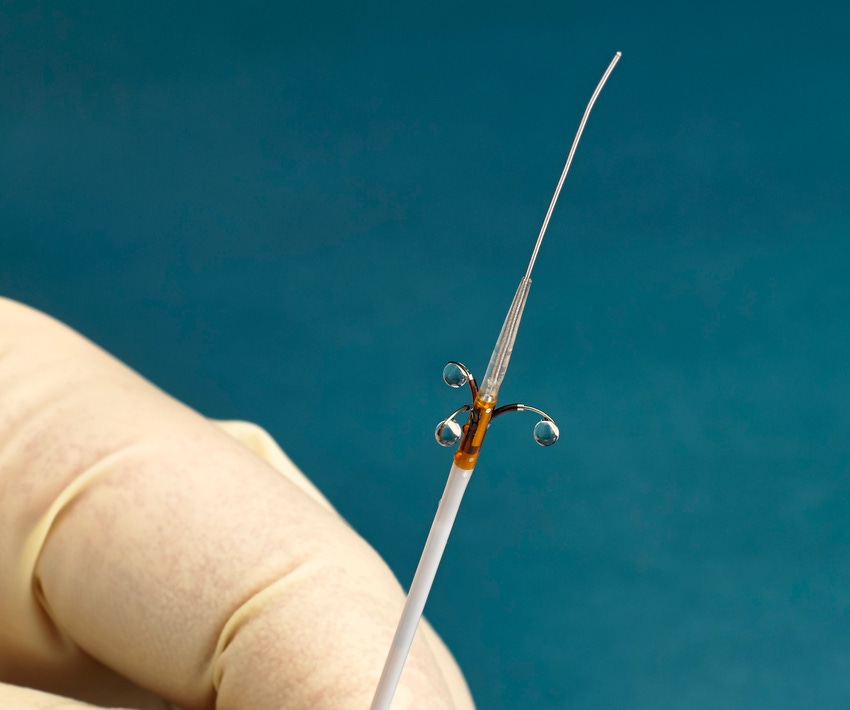
Ablative Solutions has enrolled the first patient in a clinical trial of its Peregrine System Kit, a renal denervation therapy for uncontrolled hypertension. The TARGET BP I global randomized clinical trial will look at 300 patients in up to 70 sites between the U.S. and Europe.
The Peregrine System Kit is comprised of an infusion catheter and dehydrated alcohol. The device is used in a minimally invasive procedure with the goal of deactivating the nerves surrounding the renal (kidney) arteries and thereby reducing blood pressure, said Kate Rumrill, president and CEO of Ablative Solutions
“We’ve negotiated with FDA, that if the results [of TARGET BP I] are positive that would lead to approval of our Peregrine Kit, which is comprised of both the catheter and the alcohol,” Rumrill told MD+DI. “It’s a combination kit that we would have approval for, for the treatment of hypertension.”
The Peregrine System has FDA clearance for the infusion of diagnostic and therapeutic agents into the perivascular area of the peripheral vasculature. In addition to a nod from FDA, the device has a CE mark for the infusion of a neurolytic agent to achieve a reduction in systemic blood pressure in hypertensive patients. However, the company hasn’t released the item for the sale in either place.
Instead, the firm is building data for the technology. In Europe, the company is conducting the TARGET BP OFF-MED trial - a proof-of-concept blinded, randomized sham-controlled study evaluating the safety and effectiveness of Ablative Solutions' Peregrine Kit for the treatment of patients with uncontrolled hypertension who are not taking anti-hypertensive medications. This study was initiated earlier this year and is actively recruiting.
The company also released results from the Peregrine Post-Market Study, a European multicenter open-label trial that evaluated the safety and performance of the Peregrine System Infusion Catheter, during the 2019 Cardiovascular Research Technologies (CRT) meeting in Washington, D.C. Ablative Solutions said the efficacy endpoint of the study was met, with a reduction in mean systolic 24-hour ambulatory blood pressure of 11mmHg (±14 mm Hg, p<0.001) at six-month follow-up.
“We will have more data by the end of next year with our TARGET BP OFF-MED [study],” she said. “Our commercialization is probably closer to … three years out. I really can’t get more specific than that because there are so many dependent variables. We see ourselves a year to 18 months behind Medtronic, who will be the first to get approval.”
Renal Denervation Resurgence
Trial enrollment of TARGET BP I comes on the heels of Ablative Solutions raising $77 million in a Series D financing. The financing raise showed that investors were still interested in renal denervation for uncontrolled hypertension – after many companies abandoned the space a few years back. The firms had put distance between themselves and the market because Medtronic failed to meet its endpoints in a clinical trial for the Symplicity Renal Denervation System in 2014.
Medtronic has since doubled down on its efforts in treating uncontrolled hypertension and in April of 2018 won FDA approval for a pivotal trial evaluating the Symplicity Renal Denervation system.
Shortly after Dublin-based Medtronic revealed it was re-entering the scene to treat uncontrollable hypertension, Otsuka Holdings made a play to enter the space and acquired ReCor Medical for an undisclosed sum.
The acquisition came just days after Palo Alto, CA-based ReCor Medical received approval from FDA for a pivotal trial of Paradise, an ultrasound renal denervation system. Enrollment for the trial began in October of 2018.
Rumrill commented on the recent surge in activity for the market. She said it will be the data from clinical trials that will ultimately be the determining factor for renal denervation used to treat hypertension.
“I think between [Medtronic], ReCor and our early results here with the post-market study, we’ve all been able to show that renal denervation is a safe and what appears to be an effective treatment for patients suffering from hypertension,” she said. “Granted we all are continuing to do the large randomized control trials now and three of us have those studies ongoing. Those results will be the final proof that we can kind of raise the flag and say we are correct in our assumptions.”
About the Author(s)
You May Also Like




