Abbott Has More Trouble to 'Absorb' with Bioabsorbable Stent
April 14, 2017
Abbott said it will monitor bioabsorbable stent implantations in Europe, following a high rate of heart attacks in European Absorb GT1 patients, plus a separate FDA safety alert regarding adverse events associated with the device.
Nancy Crotti
A high rate of heart attacks in European patients following implantation of its Absorb GT1 bioabsorbable drug-eluting stent has prompted Abbott Laboratories to begin monitoring surgeons' implantation techniques.
Absorb has also been implicated in reported deaths of nearly 20 U.S. patients in 2016 and 2017, according to FDA's adverse event database. Several of those reports, which were submitted by either Abbott or a healthcare provider, mentioned stent-related blood clots (stent thromboses) as a possible contributing factor. A few occurred within a day or two of discharge from the hospital.
The scrutiny over Abbott's bioabsorbable drug-eluting stent comes at a time when the company is also faced with a FDA warning letter related to products it inherited earlier this year through its St. Jude Medical acquisition, plus a new twist in its deal with Alere Inc.
|
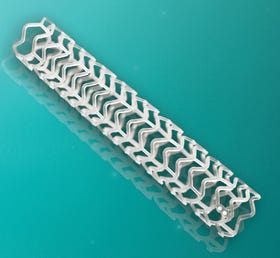
Abbott's fully bioabsorbable drug-eluting stent, Absorb, was approved by FDA in 2016. |
The FDA analysis also revealed a 1.9% rate of developing blood clots with Absorb, versus 0.8 percent within the Xience stent at two years. These adverse events were more likely to occur in patients who had Absorb placed in smaller blood vessels, the agency said.
Abbott issued a global advisory in 2015 against placing Absorb stents into very small blood vessels. The advisory suggested that healthcare providers use quantitative coronary angiography or intravascular imaging to accurately measure vessel size. There is no evidence connecting small-vessel placement of Absorb and patient deaths, according to Chuck Simonton, MD, chief medical officer for Abbott Vascular.
"The finding, that influenced our (instructions for use) label, was that there were numerically more stent thromboses in the small vessel group compared to Xience, though the difference was not statistically significant," Simonton told Qmed. "There was also no significant difference in mortality or myocardial infarctions."
The small-vessel advisory in Absorb's instructions and in the field safety notice to CE-mark countries in 2016 advised practitioners to watch for signs of thrombosis, according to Simonton. For the actual thromboses in patients who received Absorb during the U.S. pivotal trial, "all were successfully treated, and there were no deaths, including in the small vessels," Simonton said.
Abbott denied a Seeking Alpha report that it was pulling Absorb from the European market.
Nancy Crotti is a contributor to Qmed.
[Photo credit: Abbott Laboratories]
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
