May 5, 2017
Outset Medical's new technology and self-serve model could bring dialysis care into the consumer-product era of healthcare.
Amanda Pedersen
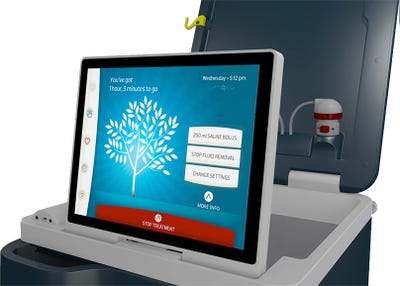
Outset Medical has designed a new dialysis machine that takes a single-serving, dialysis-on-demand approach to dialysis care.
Billions of dollars are spent every year on dialysis treatments, and yet the technology used to provide these treatments hasn't changed a whole lot over the past 30 years.
"This is one of the largest areas of healthcare that has been the least touched by new technology and service model innovation," Leslie Trigg, CEO of Outset Medical, told Qmed. "Patients today in the clinic are still served in essentially the same way they were back in the 1980s."
Outset Medical saw an opportunity to introduce a new type of dialysis technology and service model aimed at cost reduction for providers, and a better care experience for patients. The resulting product is a new kind of dialysis machine that could be compared to the Keurig coffee maker in that it offers a single-serving, dialysis-on-demand approach.
"It's sort of the K-Cup of dialysis," Trigg said.
The San Jose, CA-based company just raised $76.5 million in series C equity funding to expand the commercial introduction of its Tablo hemodialysis system in acute and chronic care markets in the United States. A new investor to the company, funds advised by T. Rowe Price Associates Inc., led the round, which also included participation from existing investors Fidelity Management & Research Company, Partner Fund Management LP, Warburg Pincus, Perceptive Advisors, and The Vertical Group.
New consumer technologies are beginning to influence the way medical device developers design products and designers are increasingly taking a user-centered approach to new projects. Outset Medical recognized that trend and wanted to design the Tablo system to look and feel more like a consumer product than a medical device.
"We saw an opportunity to produce the consumer product version of something that, historically, has been very complex," Trigg said.
While conventional dialysis machines are terrific in terms of durability and reliability, she said, those systems were primarily designed for professional caregivers.
"We're really designing for that everyday person, the dialysis patient. We wanted to design something that was accessible, and not intimidating, and didn't require any memorization or mental math," she said. "We wanted something that was automated, and would provide an experience beyond the technology."
The second differentiating aspect of the Tablo system is that it only requires an electrical outlet and tap water. Conventional dialysis machines that are used in most clinics today have to be hooked up to a fairly large, sophisticated water-processing facility that fit behind the dialysis clinics. They're usually rooms about a thousand-square-feet in size where tap water is centrally purified, and dialysate (the dialysis fluid) is made in large vasts and sent through plumbing in the walls of the clinic to the dialysis machines.
The Tablo system can be connected directly to tap water, and is designed to make the patient's prescription dialysate in real time, during the treatment.
"It has the potential to really enable providers to think more broadly about where they provide dialysis," Trigg said. "When you don't need an extra thousand-square-feet of space for a water purification facility, you can think more creatively about where you place your clinic."
For example, she said, a clinic might be placed inside a retail location to offer a more convenient, smaller environment for patients to go for dialysis, as opposed to a larger, more conventional, dialysis clinic.
Tablo is also the first hemodialysis system to have received two-way data transmission clearance from FDA, Trigg noted. In other words, the system is designed with the ability to send treatment data wirelessly after each treatment to the cloud, where it can be directly pulled down into the provider's electronic medical records (EMR) platform. The system is also capable of receiving data, so the company can send wireless software updates and, in the future, prescription data from the EMR.
"We also have a pretty sophisticated data analytics platform behind it, so we can do remote monitoring of the system, and we do remote diagnostics, which makes the service and repair function pretty efficient," Trigg said.
The Tablo system is CE-marked and FDA-cleared for use in acute and chronic care settings. The company is also sponsoring an investigational device exemption trial to expand its label indication to include at-home dialysis.
Amanda Pedersen is Qmed's news editor. Contact her at [email protected].
About the Author(s)
You May Also Like


