What Medtronic Bought on Its $1 Billion Shopping Spree
June 19, 2015
Medtronic's acquisitions mostly focus on new technologies--the most recent involving $110 million for handheld video laryngoscopes used to better intubate patients. Find out 11 companies that Medtronic has acquired or invested in this year.
Chris Newmarker
Updated November 24, 2015
During much of 2014, Medtronic CEO Omar Ishrak insisted that the company moving its headquarters to Ireland through a merger with Covidien was a good thing. While pundits speculated that the company would slow down its M&A activity following the deal, Ishrak stressed that the company would continue to invest in U.S.-based industry.
Medtronic appears to have made good on that promise when it comes to acquiring young companies with innovative technologies, with more than $1 billion in acquisitions and investments announced since Medtronic closed on the $48 billion Covidien deal in January.
Medtronic has the money to continue buying, too: It has $6.5 billion to $7 billion in free cash flow this year, chief financial officer Gary Ellis told analysts during an early June conference call. Ishrak made it clear that he's interested in snapping up new technologies.
"Using extra access we have to that cash, we stated that we're going to look at early technologies in the U.S. primarily where there maybe opportunities which we haven't been able to participate into the degree that we'd like to, to create a longterm technology pipeline of early stage technologies that we think can make a difference," Ishrak said in the call, transcribed by Seeking Alpha.
Here are 11 technologies Medtronic has acquired or invested in so far:
|
Aircraft Medical's McGrath Mac (Image courtesy of Aircraft Medical) |
November 2015: Expanding the Endotracheal Tubes Portfolio
Medtronic announced that it has acquired Aircraft Medical, a Scottish company that makes handheld video laryngoscopes used to better intubate patients. The $110 million deal expands the portfolio of endotracheal tubes that Medtronic's Covidien division manufactures. The tubes address difficult airways and prevent respiratory distress and arrest.
There may be a demand for such tubes, because post-surgical respiratory failure is the second-most-frequently-occurring preventable safety-adverse event, according to the 2013 Health Grades report on physicians and hospitals. It is quickly turning into the third-most costly hospital inpatient expense in the U.S., according to a National Center for Biotechnology Information report.
September 2015: A Candy Wrapper for Stent Retrieval
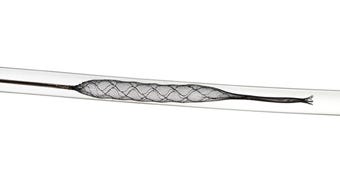
Medtronic has spent $100 million in cash to acquire Campbell, CA-based Lazarus Effect and its nitinol "mesh cover" technology that folds over a stent retriever device during clot retrieval and "candy wraps" the stent with the clot inside, Medtronic announced September 28.
|
The Lazarus Effect's cover technology (Image courtesy of Lazarus Effect) |
Medtronic considers the Lazarus cover technology to be complementary to its Solitaire stent retriever platform. "Lazarus Effect's 'mesh cover' technology complements our ischemic stroke portfolio, and further enhances our Neurovascular business's ability to deliver next generation technologies," Brett Wall, president of Medtronic's neurovascular division, said in a news release.
August 2015: 3-D Mesh for Repairing Cerebral Aneurysms
Medtronic said on August 31 that it will spend $150 million in cash to purchase Menlo Park, CA-based Medina Medical, a company Medtronic already had an ownership stake in. Medina Medical's major technology is the Medina embolization device, a 3-D self-expandable mesh that provides a scaffold across the neck of an aneurysm neck. It is able to conform to the shape of the aneurysm, reducing blood flow in the process.
August 2015: Transcatheter Mitral Valve Replacement Technology
Add Medtronic to the list of medtech companies that have invested hundreds of millions of dollars to acquire transcatheter mitral valve replacement technology. Medtronic announced in August that it will spend $458 million to acquire privately held Twelve Inc. (Redwood, CA).
The deal comes on the heels of other large medical device companies buying transcatheter mitral valve replacement device technology. Abbott announced last month that it was spending $225 million to fully acquire Roseville, MN-based Tendyne Holdings and its minimally invasive mitral valve replacement technology. A few weeks before, Edwards Lifesciences said it was paying up to $400 million to acquire Irvine, CA-based CardiAQ Valve Technologies.
All of the acquisitions--worth more than $1 billion combined--suggest that competition is heating up when it comes to providing technology for minimally invasive mitral valve replacements, which seem to be a useful option for people too weak for standard surgery.
July 2015: RF Tags for Preventing Surgical Mishaps
|
This surgical gauze is embedded with an audio frequency (RF) tag used as part of the RF Surgical Systems' RF Assure Detection System. (Image courtesy of RF Surgical Systems Inc.) |
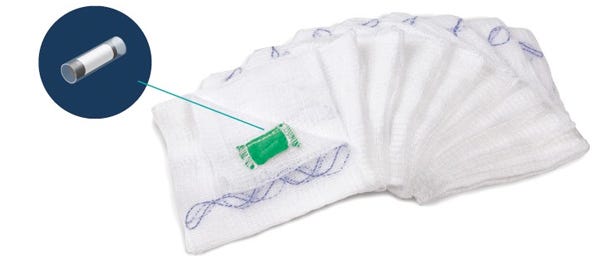
Medtronic announced July 13 that it has signed an agreement to buy RF Surgical Systems Inc., which has RF tag technology to warn surgical teams of items left in patients. Carlsbad, CA-based RF Surgical Systems has a proprietary detection system that uses a low radio frequency (RF) signal to track and detect surgical gauze, sponges and towels embedded with RF tags. The system is able to detect the items through blood, dense tissue and bone.
The system is meant as an adjunct to manual counting methods in the operating room, further insuring that surgical items are not left inside a patient after wound closure. The RF Surgical Systems Inc. technology fits in well with Medtronic's "economic value strategy," in which Medtronic is not only providing devices but also services to help health providers reduce costs and improve patient outcomes.
Medtronic spent about $235 million on the deal.
June 2015: CardioInsight's Heart Monitoring Vest
|
An illustration of the Ecvue vest, as shown on CardioInsight's website |
Under Obamacare, there is a big push for U.S. health systems to better manage patient populations to prevent more costly health problems from cropping up down the road. Better tracking of chronic conditions such as heart disease and diabetes is one solution.
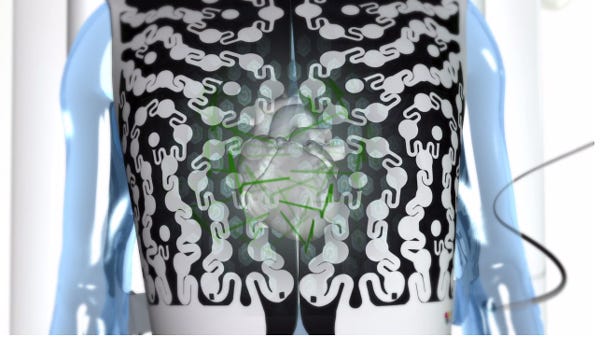
The $93 million CardioInsight acquisition, which Medtronic announced June 19, has an intriguing product in the area: a single-use, disposable multi-sensor "vest" to capture electrical heart signals from the body surface. CardioInsight also has software that turns the data into epicardial 3-D electroanatomic maps and virtual electrograms. The Ecvue vest could be used inside or outside of an electrophysiology lab.
"This investment aligns with our goal to deliver breakthrough technologies for patients who have atrial fibrillation and other arrhythmias," Reggie Groves, vice president and general manager of Medtronic's AF Solutions business, said in a news release.
June 2015: Complementary Aortic Repair Technology
Aptus Endosystems, which Medtronic is acquiring for $110 million, has technologies for endovascular aneurysm repair (EVAR) and thoracic endovascular aneurysm repair (TEVAR). Its Heli-FX and Heli-FX Thoracic EndoAnchor systems include an endovascular-deployed anchor designed to attach a variety of aortic endografts to a vessel wall.
"The Heli-FX and Heli-FX Thoracic EndoAnchor systems strongly align with our strategy to invest in treatments that address complex aortic disease with a vision to treat disease of the entire aorta," Daveen Chopra, vice president and general manager of Medtronic's Aortic business, said in a June 19 news release.
June 2015: Arsenal Medical's Foam to Stop Internal Bleeding
|
A scanning electron micrograph (SEM) of Arsenal foam (Image courtesy of Arsenal Medical) |
Medtronic is also showing a great deal of interest in materials innovations. It announced Wednesday that it is investing an undisclosed amount of money in Arsenal Medical's Arsenal AAA LLC and its proprietary polymer based foam that has shown potential to stop leaking around an endograft placed inside a weakened, bulging artery.
Arsenal Medical (Watertown, MA) counts MIT professor Robert Langer among its founders. It has developed in-situ forming foams to treat acute hemorrhage and other critical clinical conditions including vascular injury and repair.
"The investment will allow Arsenal AAA to utilize its expertise in foam technologies to address persistent endoleaks that can occur after endovascular aneurysm repair (EVAR) procedures," said Daveen Chopra, vice president and general manager of Medtronic's Aortic division, said in a news release.
April 2015: Moving Into Diabetes Care Management
Health providers' increased interest in better managing health in populations means that medical device manufacturers such as Medtronic are becoming service providers. Medtronic officials no longer want the company be just a pacemaker manufacturer or an insulin pump maker; the company is becoming a chronic disease management company.
It makes sense, then, that Medtronic announced in April that it was acquiring Diabeter, a Netherlands-based diabetes clinic and research center that is providing comprehensive and individualized care for children and young adults with diabetes.
"This acquisition marks Medtronic's first entry into a diabetes integrated care model approach and signifies that Medtronic Diabetes is more than pumps and sensors - we are a holistic diabetes management company focused on making a real difference in outcomes and cost," Hooman Hakami, executive vice president and president of the Diabetes Group at Medtronic, said in a news release.
March 2015: Sophono's Magnetic Hearing Implants
Medtronic announced in March that it was buying Boulder, CO-based Sophono and its minimally invasive magnetic hearing implants technology for an undisclosed sum.
The magnetic bone conduction hearing implants were already available in 42 countries and had been implanted in more than 4000 patients, Medtronic said at the time.
Febuary 2015: Neuromodulation Innovation
Neuromodulation remains a big, growing business in the new Medtronic, pulling in about a half a billion dollars in revenue for the quarter ended April 14. That was about one fourteenth of total revenue.
Medtronic announced in February that it had spent an undisclosed sum to buy Advanced Uro-Solutions (Elizabethton, TN), a privately-held developer of neurostimulation products for the treatment of bladder control issues.
The Nuro percutaneous tibial nerve stimulation system has FDA 510(k) clearance to treat patients with overactive bladder (OAB) and associated symptoms of urinary urgency, urinary frequency and urge incontinence. Medtronic said at the time that it was preparing to launch the Nuro in the U.S. within the next 12 months.
"Medtronic continues to invest in fully-implantable bladder control and bowel control therapies, and the addition of the Nuro system to our existing portfolio of products will introduce more people suffering from bladder control issues to the benefits of neuromodulation," Linnea Burman, vice president and general manager, gastro/urology therapies at Medtronic, said in a news release.
Learn more about cutting-edge medical devices at BIOMEDevice San Jose, December 2-3. |
Like what you're reading? Subscribe to our daily e-newsletter.
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like