March 29, 2016
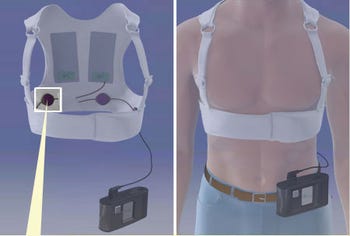
The wearable cardiac defibrillator vest may be a good option for patients who cannot tolerate an implant.
Nancy Crotti
The American Heart Association, based on more than 100 studies of patients using the LifeVest Wearable Cardioverter Defibrillator. The group published an advisory in its journal, Circulation, its first on the devices.
Zoll Manufacturing of Pittsburgh, PA, makes the only defib vests on the market, for use by adults and children, but there are other devices in development, according to an AHA spokesperson.
Zoll won FDA approval for its first vest in 2001 and began marketing it in 2002. The company declined to comment for this story.
The advisory described patients who are not good candidates for implants as those who recently had blood flow improved through surgery or angioplasty and those who have an active infection or unknown prognosis. Heart rhythm abnormalities are common in those patients as well as in those with cardiomyopathies. Sudden cardiac death accounts for more than 300,000 patient deaths in the United States annually, the AHA noted.
"For many of these patients, the risk for life-threatening rhythm abnormalities may be temporary, so the wearable cardiac defibrillator could be a short-term alternative to an ICD, which is permanently implanted in patient's chest," said Jonathan Paul Piccini, MD, lead author of the advisory and a cardiac electrophysiologist at Duke University Medical Center in Durham, NC, in an AHA statement.
Heart failure patients waiting for a transplant may also be good candidates for the vest, the advisory says. However, patient compliance has been known to stumble due to the vest itself, fashioned like a fishing vest in the back with a broad strap across the chest.
It's uncomfortable, Piccini said, adding that may change as the technology improves.
The advisory fell short of recommending systematic use of wearable defibrillators and said that randomized clinical trials would be needed to determine if they lead to improved outcomes.
"Although a growing number of patients are being prescribed wearable cardiac defibrillators by their doctors, there have been very few well-designed and completed studies of these devices. Wide spread use of the wearable defibrillator is not advisable because there isn't enough clinical evidence to support its use, except in a small number of patients with known life-threatening arrhythmias but for whom surgery to implant an ICD is not advised in the short-term," Piccini said.
Zoll did not respond to a request for comment on the advisory.
"As with most new medical technologies, biomedical engineers are working to make them smaller, more lightweight and less burdensome. One company is already developing a self-contained system in a large, self-adhesive plastic bandage that a wearer just sticks on their chest," Piccini said.
The AHA declined to name manufacturers, as it does not make implied endorsements of individual products, a spokesperson said. Piccini could not be reached for comment.
Learn more about cutting-edge medical devices at BIOMEDevice Boston, April 13-14, 2016. |
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like


