The Two-Year Turnaround
Left for dead not long ago, Cyberonics Inc. has roared back to life thanks to a new—but also old—focus.
November 1, 2008
MANUFACTURER OF THE YEAR: CYBERONICS
|
(click to enlarge) |
In early 2007, things could not have been any worse for Cyberonics Inc. (Houston). It was buried under an avalanche of negative publicity and underperformance. The neuromodulation company received a major setback from CMS, which denied reimbursement for the depression indication of its VNS (vagus nerve stimulation) Therapy System. As a result, its financials suffered, and auditors expressed concern about future viability. Creditors went after the company in court. To make matters worse, the year before, the firm had come under fire for the timing of stock-option grants to top executives, news that generated a lot of bad publicity. Its CEO and CFO resigned. Rumors abounded that corporate raider Carl Icahn, whose specialty is buying underperforming companies, stripping them down, and selling them off for a profit, was going to take over the firm.
In 2008, however, the outlook could not be any better for Cyberonics, given the adversity it was facing less than two years ago. A new management team came aboard in spring 2007 and refocused the company on its more profitable product applications. New hardware products were well received. Licensing deals brought in some more cash. The firm was able to settle with its creditors. All of this has added up to five straight quarters of epilepsy growth, with the last two being profitable.
For its quick, unexpected turnaround and the contributions its technology has made to healthcare, Cyberonics has been named MD&DI's Manufacturer of the Year for 2008.
Anatomy of a Resurrection
Cyberonics is one of several companies playing in the neuromodulation and neurostimulation space. It is a technology platform with huge potential. Many expect it to do for the brain and nervous system what cardiac rhythm management technology did for the heart: provide electrical charges that change a fault in the organ or system.
But Cyberonics, which was founded in 1987 by Reese Terry and Jacob Zabara, goes about this differently than its competitors, many of whom have been bought up by device-industry giants in recent years.
“Our technology is a lot less invasive than deep brain stimulation,” another common neuromodulation technology, says president and CEO Dan Moore. “We are able to have an impact on the brain without doing brain surgery. Instead, our technology makes use of the vagus nerve in the neck.”
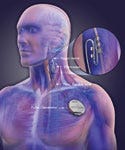
VNS Therapy works by delivering stimulation via a device, which looks and acts a bit like a pacemaker, implanted just under the skin in the left chest area. The device sends mild, intermittent electrical impulses through a lead to the left vagus nerve, which then sends signals to the brain. Each device is programmed for the individual patient, and the patient or a caregiver can initiate or abort stimulation with the use of a hand-held magnet.
Zabara, a professor at the Temple University School of Medicine in Philadelphia, hit upon the idea in 1971 while watching his wife control labor pains during childbirth via breathing exercises. He hypothesized that her breathing was making a connection to the brain via the vagus nerve, and that if this was so, then the vagus nerve could serve as a pathway to the brain to control conditions such as seizures.
Unfortunately, no one took his research seriously until the mid-1980s, when he met Terry, the holder of several patents related to pacemakers and then the vice president of technology at Intermedics. He looked into the technology on behalf of Intermedics. The company declined to pursue it, but when Terry left as a result of restructuring, he decided to license the technology and develop a product himself. He and Zabara incorporated Cyberonics in December 1987 and rounded up start-up funding.
An initial public offering in 1993, just a few months before the firm filed to get FDA approval for VNS Therapy to treat epilepsy, raised $24 million. But that looked like it wasn't going to be enough after the agency told Cyberonics that a second, confirmatory clinical study would be required. Board member Robert “Skip” Cummins, a general partner in a venture capital firm that was an early investor, took over as CEO and convinced St. Jude Medical to invest $12 million to complete the trial. (St. Jude Medical had an option to buy the company for $72 million but declined to exercise it.)
Finally, in 1997, VNS Therapy received FDA approval to treat epilepsy. Two years later, CMS granted reimbursement for the procedure. The technology had success in that field, but Cummins viewed the technology as a platform that could be used for many indications. He was so confident in its potential that he and the board rejected a $480 million buyout offer from Medtronic in 2000.
Cummins was particularly optimistic about the technology's potential for treating depression, so that became the focus of Cyberonics's R&D efforts. Early studies were positive, but preliminary results for a pivotal trial in 2002 were not. The company made changes to the ongoing trial and went before FDA in 2003 with positive results. The agency overruled an advisory panel recommendation for approval and rejected the application in 2004, but Cyberonics did not give up. It spurned another buyout offer—this one from Advanced Neuromodulation Systems for $524 million—and continued to press its case with FDA. In 2005, after receiving positive two-year study results from the company, the agency reversed itself, setting off a huge controversy in which consumer groups and some members of Congress accused the agency of improper conduct. (A Senate investigation concluded that CDRH director Daniel Schultz had overruled his scientific staff, but the decision was allowed to stand.)
But the projected windfall from the approval never happened. In early 2007, CMS denied coverage for the depression indication, meaning there would be no reimbursement for its use on Medicare or Medicaid patients. That meant that after all the efforts the company put forth and the controversy it endured, revenues from the depression indication would be minimal.
That was not the only adversity Cyberonics found itself up against. In 2006, it was revealed that Cummins and two other executives had been given stock-option grants just hours after the firm received positive regulatory news. The next day, after the news was made known, the value of the stock jumped. Critics again went after the company, accusing it of backdating or spring loading its option grants.
The company was hemorrhaging cash, having lost about $100 million in two years, and was left with just $85 million. In the company's quarterly financial reports, its auditors expressed concern about whether the company would be able to survive. In November 2006, Cummins and CFO Pamela Westbrook resigned. Wall Street presumed the company was going to be sold, possibly to Icahn, who had begun to buy up shares. Terry agreed to take over as CEO until a permanent replacement could be found.
Turning Things Around
This was the environment that Moore, a division president at Boston Scientific, walked into when he took over as president and CEO in May 2007. Why would someone from one of the biggest companies in the entire industry want to take the reins at a smaller company whose future was in doubt?
“First and foremost, it was the potential of neuromodulation,” says Moore. “My belief, having been with Boston Scientific since 1989, was that we and some other big companies had solved a lot of the issues regarding [minimally invasive surgery] in the heart and the coronary and peripheral arteries. And we solved a lot of issues around the electrical system of the heart. But the final frontier is the electrical system of the brain. We will be seeing major advances in that area in the next 5–15 years.”
And he saw Cyberonics in 2007 as similar to Boston Scientific in 1989: Each had about $100 million in annual sales at the time, and each was poised to become a major player in a field that was about to undergo stunning growth, thanks to technological innovation.
He liked that Cyberonics had a foothold in the business for 10 years. VNS Therapy as a treatment for epilepsy was proven to work, and it had gone through four generations of models.
So Moore thought the best way to start was to focus on growing the epilepsy application, which had lagged in sales in recent years as the firm had devoted more of its resources to treating depression. This might be one of the quickest ways to right the income statement, which had been $50 million in the red each of the previous two years. “Many people here believed that we could grow the epilepsy business if we focused on it,” he says. “We determined that the market was underserved, and that new technology on the hardware and software sides could help us grow it.”
And grow it did, as the epilepsy business has had four straight quarters of double-digit growth—if you count a rounding up from 9.9% in one quarter. Growth has been particularly strong outside the United States, where the firm increased its focus. In 2007, epilepsy revenue grew 8.3% domestically and 21% internationally.
Management, including a new CFO, Greg Brown, then had to sort out what to do with the depression indication. “We had put a lot of effort into it, but we could not continue at the pace we were on,” says Moore. “If you're not getting reimbursement but you're continuing to spend so much money on it, it's time for a change.” So management made the painful decision to severely cut the funding of depression operations and shift personnel and resources to epilepsy operations. Layoffs, unfortunately, were necessary.
But that turned out to be a smart move, says Thomas Gunderson, managing director and senior research analyst with Piper Jaffray & Co. (Minneapolis).
“I think they grew too far, too fast, and had an infrastructure that was too large relative to the business that they could do,” he says. “They hit a wall with reimbursement, repeatedly, until new management said ‘We are going to run this business for profit.' They refocused their sales force on the reimbursable product for epilepsy. And they introduced new [hardware] products with higher price points.”
|
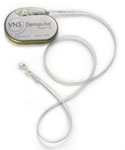
The Demipulse, which received approval in July 2007, is smaller than previous models. Releasing an upgraded part helped the company recoup some losses. |
Neuromodulation devices, like pacemakers, need hardware replaced every so often. Cyberonics received approval for a new generator, the Demipulse, in July 2007. It is smaller than previous models and has been very well received.
Each part of the company had to figure out what it could do to turn things around, and the legal department was no exception. One of its biggest challenges was dealing with bondholders to whom the company owed $125 million. After the stock-options controversy, Cyberonics had to restate earnings and was late in making some filings to the Securities and Exchange Commission. The bondholders were required to be notified within 10 days of when filings were made. They believed that, because the filings were late, Cyberonics had defaulted, and they went to court to get such a ruling.
“We did not think we had defaulted,” Moore says. “We had to provide the bondholders our 10Q submissions within 10 days of being filed, but there was nothing in the agreement about having to have them by a certain date.” In other words, Cyberonics was still providing the material within 10 days of when it was filed, even though it was filed at a later date than expected. And once it sorted out its restated earnings, its submissions were on time again.
In June 2007, a court agreed with Cyberonics. The bondholders were going to appeal, but instead of continuing to rack up legal fees, Cyberonics decided to make a deal. It agreed to push up the call date of the bonds from September 2012 to December 2011 in exchange for the bondholders dropping their appeal, and for reclassifying the debt as long term instead of short term. So the bondholders will be paid off nine months earlier. The company has already bought back more than $25 million of the bonds. And one factor that was contributing to the perception that the company would not survive has been eliminated. Today, the auditors are no longer including the dreaded “going concern” clause in their reports.
Another factor in the turnaround, says Moore, was the employees who remained. “Once we determined that we were returning to the epilepsy business, 500-plus people decided to reapply their drive to the epilepsy field. That was the first and foremost reason why we were able to be successful.” Given all the turmoil that the company has gone through in its history, it is not at all surprising that Moore inherited a very determined team.
“Because of the number of problems the company faced, anyone who was left was resilient,” he says. “We were able to use that. It speaks to the passion of the people who were here. We still had a lot of talent at the company. It's all about the team you put on the field. It's easy to make a plan. The challenge is executing the plan. And it showed when we turned in a profit in our fourth quarter [of 2007] after two straight years of $50 million losses.”
Change in Strategy
The most important decision, perhaps, was when management determined that, contrary to the mantra under Cummins, it could no longer view VNS Therapy as a platform technology from which it could develop many indications. It simply did not have the resources to pull that off. So it had to figure out what to do with each potential indication.
“We identified all the potential applications, 20 or so, and put them into three ‘buckets,'” Moore says. “The first bucket consisted of indications for neurology and neurosurgery. The second bucket was depression, and other indications for psychiatry. The third was other potential VNS indications, such as obesity and bulimia. We decided we would focus epilepsy and keep other neuro applications such as Parkinson's and MS. For psychiatry applications, we decided to seek a partner to continue with depression, and package some of the psychiatry indications as one group. The third bucket had many indications with market potential that excited us. But as a $100 million company, we could not address the needs of all three buckets and build a good, solid business. So we decided to spin off bucket number 2 and get partners for bucket number 3 on a case-by-case basis.”
|
Cyberonics's headquarters in Houston. The facility holds what Moore describes as a very resilient team. |
The first action with bucket number 3 occurred in December 2007, when Cyberonics struck a deal with Ethicon Endo-Surgery, a Johnson & Johnson company, for the obesity indication. Ethicon Endo-Surgery received the rights to Cyberonics's patents on vagus nerve stimulation related to treating obesity, diabetes, and hypertension in overweight people. Cyberonics received $9.5 million and future royalties.
Cyberonics recently announced that it was unsuccessful finding a partner for depression, but Moore is optimistic. “We like the depression indication overall,” he says. “It's a big potential market, and VNS is still the only FDA-approved device for treating depression.” Indeed, the company is continuing with two postmarket studies that were a condition of FDA approval for the depression indication. It hopes that they will turn up additional evidence that VNS Therapy is an effective treatment for depression; such evidence will be needed to get CMS to change its mind.
In keeping with the strategy, bucket number 1 got larger in December, after Cyberonics licensed patents from Southern Illinois University for vagus nerve stimulation for treatment of stroke and traumatic brain injury.
A Bright Future
Going forward, the top priorities are growing the epilepsy business and continuing to develop other neuromodulation applications.
|
The VNS device shown here features Demipulse hardware. Cyberonics only has one product, but that product has many potential applications. |
“The next step for us initially is to remain the leader in medical device treatment of epilepsy,” Moore says. While international growth has been strong, he says, it can be even stronger. VNS Therapy was recently approved for treatment of epilepsy in China, and the company is working on getting approval in Japan.
U.S. numbers provide room for growth too, Moore says. About 2.7 million people in the United States have some form of epilepsy. About half of those have types of epilepsy that are within the current FDA-approved labeling for VNS therapy. Drug treatment is always tried first, and it fails in about one-third of patients. That means that more than 400,000 U.S. patients could use the therapy. “In 11 years, we've reached about 40,000 patients, so we only have 10% of our potential market—less if you consider that of the 125,000 newly diagnosed epilepsy patients each year, at least 13,000 could benefit from VNS Therapy. We were successful last year, but we still only reached about 6000 patients, both new and those needing generator replacement.”
|
Gunderson agrees that the firm's turnaround is sustainable. “As remarkable as what they've done is, it was a quick fix. They cut costs and got a bit of luck with the hardware replacement cycle,” he says. “They will have to show good growth year over year in the epilepsy business, and spend more time developing other indications for neurology and related fields. But I think they can do it.”
So, while it may seem as though Cyberonics has retrenched by narrowing its focus to epilepsy, in reality, its growth potential is probably higher than it was when it was spreading itself over a number of different applications.
There is much room for technological advances, as well, Moore says. “A lot of technology can be brought to bear to treat epilepsy,” he says. “With technology advances, we may be able to alert patients that they are about to have a seizure and help them initiate therapy. Future devices may notify a caregiver. New devices may count seizures, leading to better patient care. There are a number of potential businesses within epilepsy that do not exist today. There is a lot we can do to help more patients with epilepsy. And we have a team that is dedicated to making it happen.”
Copyright ©2008 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like




.png?width=300&auto=webp&quality=80&disable=upscale)
