Simple Approach Leads to Singular Success
Roche Diagnostics puts its many strengths into personalized medicine. Its objective is to change healthcare from the inside out.
November 1, 2006
MANUFACTURERS OF THE YEAR: ROCHE DIAGNOSTICS
|
Lonnie Shoff, senior VP of molecular diagnostics and applied science |
An analysis of in vitro diagnostics (IVDs) from Merrill Lynch released in 2004 says that Roche Diagnostics (Indianapolis) is a powerhouse in the IVD market. It is the first company that has been able to make personalized medicine a reality. Its revolutionary products, however, do not come from radical ideas or luck. Instead, Roche has a strategic vision that enables it to focus its efforts to push forward incredible technologies. And in talking to Roche leaders, it becomes clear that such success is based on some very simple principles.
Roche's main goal is to “identify and develop areas with unmet needs,” says Lonnie Shoff, senior vice president of molecular diagnostics and applied science. “We want to improve standards of care in areas where patients aren't being served.” Shoff explains that to find those unmet needs Roche has to be very active in the industry. “We have a lot of interaction with the market, through our advisory board, and through workshops, conference sponsorship, and our work with local laboratories.” Roche works with several labs around the country and dubs them the Molecular Centers of Excellence. “Through these centers we can share information freely, and they help us identify the needs of the marketplace.” Shoff says that Roche's real strength is finding niche markets and fulfilling health needs for those patients.
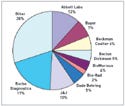
And because the company has 19% of the total IVD market—more than any other single company—it's obvious that Roche is meeting its goals (see Figure 1).
“These guys get it,” says Bruce Jackson of the investment firm Miller Johnson Steichen Kinnard Inc. (Minneapolis). “If I had to pick one thing that sets Roche apart, it's strategic vision. They understand the value drivers in IVD testing, and they look for ways to enhance that value proposition to the customer.”
Indeed, the company is present in almost all aspects of IVD and is dominant in the glucose monitoring and molecular diagnostics fields. But aside from its goal to meet the needs of niche markets, Roche seems to do something more: it spreads itself out. And it uses its considerable resources to form alliances with other technologically advanced companies. For these reasons, MD&DI has selected Roche Diagnostics as one of its Manufacturers of the Year.
|
The AmpliChip CYP450 is used in many laboratories across the United States and in Europe. |
A Difficult Market
Jackson says that such success is hardly easy to achieve. “IVD has been a challenging market for a long time, ever since diagnosis-related groups were introduced in the United States, which turned the lab into a cost center instead of a profit center,” he says.
As a result, companies have to compete in an environment where cost containment is the norm. “That means demonstrating how more testing can reduce total cost by reducing length of stay, for example,” explains Jackson. “Or how pharmacogenomic (e.g., personal care) testing can personalize drug therapy for some people so that it is more effective while reducing the side effects and the risk of an adverse event. The new cost problem is utilization driven, not price driven.” Roche Diagnostics, he says, has met this challenge by making healthcare resources more efficient.
“With the healthcare environment increasingly focusing on cost containment, it is a struggle to get payers to realize the value of diagnostics,” says John Ridge, director of reimbursement at Roche. Ridge acknowledges that with the changes made in the reimbursement arena, “gaining payer approval has become as important as securing regulatory approval for new medical products.”
According to Ridge, the only way to meet reimbursement challenges is to actively pursue reimbursement. “A key part of any reimbursement strategy is to involve our reimbursement services department early on in the product development process to make sure that barriers and opportunities are identified and addressed before a product is ready to be released to the market.”
To do so, Roche has implemented a premarketing program that combines reimbursement and scientific affairs resources. “We have to make sure that payers have the data they need to establish payment policies for diagnostics tests,” says Ridge.
|
Figure 1. Market shares in IVD for 2005. Source: Kalorama Information. |
A Wide Footprint
Part of Roche's strategy to overcome market pressure has been to widen its diagnostic footprint. Because of its diverse nature, the company has been able to take revenues from slow but cash flow–rich base markets such as clinical chemistry and immunodiagnostics and deploy that capital into faster-growing markets such as molecular diagnostics. “Being the market leader in molecular diagnostics with such a strong presence in mature markets gives Roche more solidity to engage in less-developed markets and riskier projects,” says marketing manager for molecular diagnostics Brigitte Fernandes-McAlear. Roche's investments in polymerase chain reaction (PCR) have enabled it to set the gold standard for genomics work. “When Roche decides to enter a new market, everyone takes notice. It took 15 years, but they built PCR testing into a billion-dollar business,” says Jackson. In July 2005, Roche opened the world's largest manufacturing facility for PCR-based products in Branchburg, NJ. Many of its most well-known technologies have come from the company's solid base in PCR.
In addition, Roche has been able to gain premium market share through collaborations with companies such as Affymetrix (Santa Clara, CA) to further product development. Affymetrix has been integral in developing what is perhaps Roche's best-known product, the AmpliChip CYP450. “This collaboration brings together state-of-the-art technologies,” Fernandes-McAlear says. “The AmpliChip CYP450 test uses Roche Diagnostics' Nobel prize–winning PCR technology, and it is powered by an Affymetrix high-density microarray platform.”
The AmpliChip CYP450 tests the metabolism profile of a patient so that doctors can gauge medication type and dosages that will maximize benefits and minimize side effects. It supplies data on gene variations for the 2D6 and 2C19 genes, which play a role in metabolism of about 25% of all prescription drugs. Affymetrix provides the microarray system for the test. The two companies have an 18-year licensing agreement worth $70 million. The AmpliChip CYP450 gained 510(k) clearance in January 2005 in conjunction with the Affymetrix system.
According to Roche, the AmpliChip CYP450 test was the first pharmacogenetic and microarray test to obtain FDA clearance. “We are forging new territory,” Shoff says.
According to the company, Roche has played a large role in educating psychiatrists, sponsoring CME programs, and supporting various clinical studies to strengthen the clinical validity of using the AmpliChip. “These new tests provide actionable health information that physicians did not use in the past,” Fernandes-McAlear says. “Therefore, physician education, through market development, is a critical success factor in realizing the full potential of molecular testing. Both laboratories and manufacturers have a key role to play in market development.”
Development is under way for clinical applications of an AmpliChip test of the p53 gene. The p53 is one of a family of genes called tumor suppressors, which code for proteins that prevent damaged cells from reproducing. Mutations of the p53 gene are found in virtually all tumor types. By identifying mutations that affect p53 function and activity, the AmpliChip p53 test may one day help physicians select the anticancer medicines best suited to their patients' needs. “This is still in development, but it really brings new and exciting technology to the area,” says Shoff.
In addition to entering new markets, Roche is building its reputation in already established markets. In 2005, it introduced 20 new products and plans to release at least 11 products in 2006. Some of the highlights include devices in the diabetes care market such as the Accu-Chek Pocket Compass 3.0, which includes software for mobile diabetes self-management. According to the company's 2005 business report, the device features a bolus calculator module that enables users to calculate their bolus insulin requirements on the basis of current blood glucose, estimated food intake, and several personal parameters.
The company is also introducing diagnostic products such as the Cobas 6000 series, which consolidates clinical chemistry and immunochemistry testing for medium- to high-workload laboratories. It has up to seven configurations, using Cobas c501 and Cobas e601 modules. In molecular diagnostics, the LightCycler SeptiFast test, a device that diagnoses sepsis-causing pathogens directly from blood, has gained a CE mark.
|
A Roche employee checks the fluidic tubes on a rig used to prepare samples for PCR testing. |
Time Is on Their Side
Roche's most recent product, however, is not actually ready for clinical applications; it's still in the research stage. The Genome Sequencer 20 can decipher more than 20 megabases in five hours on a single instrument. Like the AmpliChip, the genome sequencer is the product of a strategic alliance with its inventor, 454 Life Sciences (Branford, CT). It is being used for various sequencing applications, from unknown viruses to ancient DNA (such as that of a wooly mammoth). The company also has plans to release an application for an ultradeep sequencing of PCR products. The kit will help identify somatic mutations in complex cancer samples. It also can be used for high-confidence single nucleotide polymorphism discovery on a population level, a process known as medical resequencing.
Although at this point the sequencer is strictly for research purposes, the company has high hopes that it could one day be used in clinical applications. “There is no doubt that sequencing has a future in diagnostics,” says Shoff.
Still, says Shoff, the research market is key to the future of diagnostics. “Our main goal in diagnostics is to provide researchers with tools that are more innovative than what is currently on the market. Such products could make their jobs easier and more efficient.”
To provide that type of innovation, she says, companies must be able to put in the time. “One thing about being the market leader is that we are able to have the patience to invest in research diagnostics.” Those research sectors could one day be focused into clinical diagnostics, but they still need quite a bit of investment.
Conclusion
Roche's ambitious plans, its ability to form alliances with innovators, and its stable capital are important factors in the company's long history of success. Jackson says that Roche Diagnostics' vision is enviable. “It is pioneering new markets like personalized medicine and rapid testing of pathogens, which are potentially high-value testing applications,” he says. “Other companies are developing similar products, but Roche will have the territory staked out, and they have the infrastructure in place to develop and service these markets.”
For Shoff, the recipe for success is less about having a strategic business plan than it is about always serving customers and working toward better healthcare. “Because we are focusing on unmet needs, we're often going to be first on the market. That is good for business, but it is also good because we are interested in doing it right.”
Shoff is passionate about the work her groups are accomplishing, including the AmpliChip and the Genome Sequencer 20. “There is a lot of awareness of personalized medicine. Being able to tailor therapy to an individual means that therapy is effective in a personal way. Clearly, the way to improve both patient outcome and economics is to give physicians tools to help them make better decisions. Personalized medicine is a tremendous way to achieve that goal.”
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like