Rapid Liquid Biopsy Could Make Cancer Treatments More Successful
The Parsortix system from Angle plc harvests circulating tumor cells and aims to turn a simple blood draw into valuable information for personalizing cancer treatment.
September 11, 2015
Marie Thibault
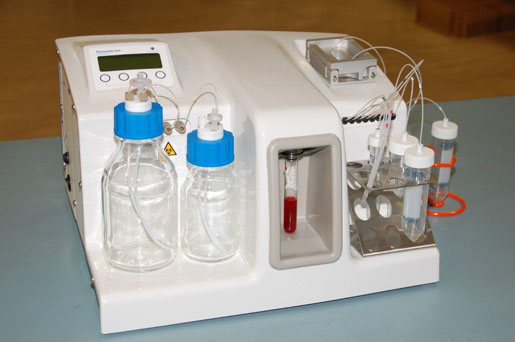
The Parsortix system from Angle plc captures and harvests circulating tumor cells.
Today, potential cancer patients often have to undergo an invasive solid tumor biopsy to find out whether a tumor is cancerous and if so, what stage their cancer has reached. This analysis might also provide other valuable information, including the cancer type and how aggressive it might be, giving physicians the ability to better tailor cancer therapies to the individual's specific cancer.
Of course, the cancerous tumor is often removed, which can help check the spread of cancer, but doesn't always rid the body of cancer. And losing the physical tumor means physicians can't just perform another solid biopsy to keep tabs on the cancer.
"What happens over time is, just because the cancer has been cut out does not mean that it's been cured . . . it may or may not be developing toward a metastatic state," says Andrew Newland, CEO of Angle plc. He explains that over time, the type of cancer could change as well, making certain therapies more or less effective as it evolves. "It's impossible to redo the solid biopsy because they've already cut the lump out . . . but they could run a blood test."
|
Newland |
That's exactly what the Parsortix cell separation system from Angle plc is designed to do. The system, which looks like a filtration system, captures circulating tumor cells (CTCs) in the blood. These CTCs, which come from primary tumors and cause secondary cancers at other locations in a patient's body, are hard to find, since there is about one CTC per billion blood cells. The Parsortix technology works by exploiting characteristics of the CTC—namely, its large size and less compressible nature. The system's patented microfluidic cassette catches and harvests the CTCs.
Newland points out that with a blood draw and the Parsortix system, a patient's cancer could be analyzed repeatedly, as often as needed. This could improve cancer treatment by allowing therapies to be targeted specifically at the individual cancer as it exists at that particular point in time.
Several academic centers, including the Medical University of Vienna, University of Surrey, and Barts Cancer Institute have been studying the Parsortix system's clinical applications for various types of cancer.
Earlier this year, the results of a small 18-month ovarian cancer patient study conducted at the Medical University of Vienna were presented. The study found that the Parsortix system led to 100% specificity in primary epithelial ovarian cancer—meaning there were no false positives—78-80% sensitivity with 7 RNA markers, and 100% sensitivity with 30 RNA markers, a much higher rate than a competing technology.
Newland envisions Parsortix having a clinical application in ovarian cancer that would help triage patients. About 11% of the U.S. women who undergo surgery for abnormal pelvic masses have ovarian cancer and Parsortix could help pick those patients that have a high risk of cancer in order to ensure the appropriate surgical approach is taken.
Newland explains, "If she's got ovarian cancer, they need a very, very specialized type of surgeon who is experienced in cutting out ovarian cancer. It will be spread all over the pelvic area and it will probably take a six hour operation of very, very precise work by an expert to probably clear away all the traces of the cancer. . . If a general surgeon starts a one hour operation and finds out that he's actually got an ovarian cancer patient, then unfortunately the likelihood for that patient is a very poor outcome . . ."
Plans for a larger study for the ovarian cancer clinical application are being finalized across the United States and Europe and is expected to conclude by the end of 2016. Newland expects Parsortix to be in clinicial use in Europe before the end of 2016 for patients that potentially have ovarian cancer. Even better, there is already Medicare reimbursement coverage in place for ovarian cancer tests, which might ease the technology's introduction in the United States. Work on other diseases, like breast, prostate, colorectal, and pancreatic cancer, is also being conducted at other centers.
There are a number of competitors, but a comparison against these other technologies in Angle's corporate presentation highlights that none satisfy all the criteria that Parsortix achieves: simple, inexpensive, able to capture all cancer types and mesenchymal CTCs known to be part of metastasis, easy harvest, and high purity of harvested cells for analysis.
Translational researcher James Reuben, PhD, MBA is a CTC expert and professor of hematopathology at MD Anderson Cancer Center in Houston. He has experience with Parsortix as well as competing technologies, and says that one advantage of the Parsortix technology is the easy process, requiring one throughput to capture CTCs, which can then be analyzed further—"we can image, we can stain," he says.
Parsortix is available for research applications now and has CE Mark for the clinical market in Europe. Work on the FDA process to get approval for clinical use in the United States is ongoing. Unlike many of its competitors, which have chosen to eschew the FDA for the CLIA approach, Angle plans to sell the Parsortix to customers for use in their own lab or setting, as opposed to requiring customers to send in samples and wait for a result from Angle.
Newland says sales have already begun to researchers for research use. Eventually, once regulatory approvals are secured, the main focus will be on sales of the system for clinical applications.
Learn about trends in medical design innovation at the MD&M Philadelphia conference , October 7–8, 2015. |
Marie Thibault is the associate editor at MD+DI. Reach her at [email protected] and on Twitter @medtechmarie.
[Images courtesy of ANGLE PLC]
About the Author(s)
You May Also Like



