Process Validation Prerequisites 101
Medical device companies must meet a predefined set of requirements to ensure successful process validation.
VALIDATION
|
Photo by D. GUZMAN & H. TORRES |
Developing a medical device is a lengthy process. Prior to commercial distribution, FDA requires that the manufacturing process for the product be validated. Process validation is defined as the accumulation of documented evidence that demonstrates with a high degree of assurance that a process can consistently meet its predetermined specifications and quality attributes. Not only does process validation satisfy regulatory requirements, it is good business practice to have the confidence that a manufacturing process is repeatable and will yield a quality product on a consistent basis.
Most companies understand the requirement for process validation. However, the way companies go about process validation often lacks the business-related benefits that may result from truly challenging the entire process.
For example, a company may simply repeat the normal manufacturing process a number of times, collecting routine samples, and analyzing the samples for in-process and finished-product specifications. However, this process is insufficient to demonstrate that a process is validated. Performing validation in this manner only shows that at that particular date and time, the technical aspects of the manufacturing process may or may not have been successful. The goals of validation are to demonstrate repeatability, ensure a robust process after the process validation report is approved, and obtain a business-related benefit from the process validation activity. To perform these tasks, the quality systems contributing to the technical aspects of the process must be in a state of control.
If manufacturing support systems are not in control and are not sufficiently thorough, so-called quality systems cannot be counted on to ensure the same process results. This is where the value of incorporating prerequisites comes into play.
Companies should be able to prove that they have addressed and controlled all supporting process-related variables before starting the process validation production runs.
Meeting Prerequisites
|
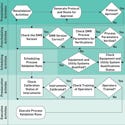
Figure 1. (click to enlarge) Prerequisites should be performed following a strict protocol to ensure robust validation and to achieve business-related benefits. |
What are prerequisites? A prerequisite is documented verification that a specific supporting quality system related to the manufacturing process being validated is as specified. A prerequisite verification is performed prior to executing the manufacturing runs.
When met satisfactorily, the prerequisite is intended to demonstrate readiness for execution of a manufacturing run. A process that is not performed the same way every time cannot be considered validated. This is the core concept behind using prerequisite verifications during process validation. Any area that could lead to variation in a manufacturing process or in the analysis of the manufacturing process should be included in the prerequisite verifications section of the process validation protocol. The following sections explore common areas that should be verified because they often lead to variation in the process.
Device Master Record (DMR) Status Verification. The DMR is a road map for the manufacturing process. In this prerequisite, the effective version of the DMR is verified as the latest approved version of the document. The version used must agree with the version listed in the approved protocol.
If the effective version created during protocol generation is the same as that used on the production floor, then it is safe to initiate the process validation production runs. If not, there is a high probability that the protocol may be inaccurate (possibly resulting in numerous failures) or that the process itself is not ready for either process validation or commercial production.
For example, during a recent process validation activity at an orthopedic device manufacturing plant, modifications were made to the DMR less than a day before the already approved protocol was to be executed.
Certain processes were modified without the knowledge and consent of the validation team. Unfortunately, the approved protocol did not state the correct directions to follow. As a result, there were numerous deviations (i.e., investigations) that needed to be documented and addressed during the execution of the process validation production runs. The company wasted time and money. But more importantly, the error led to questions about the company's compliance (i.e., the ability of the quality system to catch issues prior to and during production). If DMR status had been verified as a prerequisite, this problem would have been caught prior to executing the runs.
Operator and Test Personnel Training Verification. In manufacturing, many standard operating procedures and analytical test procedures are used. The purpose of process validation is to provide assurance of the repeatability of a process. Therefore, operators and analysts must be trained on all procedures that may affect the manufacturing and testing of the process. This prerequisite checks the training records of the operators to ensure that they have documented training on the procedures performed during process validation. Implementing proper training not only satisfies risk compliance, but it is also good business practice. Failures caused by untrained-operator errors result in additional consecutive process validation production runs.
For example, during a recent preapproval inspection of a medical device manufacturer, an investigator was reviewing the executed process validation protocol for the product being assessed. The investigator asked to see the training records for two of the employees who performed the release testing on the finished lot of product.
When given those records, the company realized that the operators had not been trained on the test procedures. This situation called into question the validity of the test results. The company had to repeat the costly and time-consuming testing, which would have been avoided by verifying training prior to execution.
Equipment and Utility System Qualification Verification. Equipment and utility systems are two of the most critical areas affecting the outcome of a manufacturing process. It is important to verify that the commercial equipment and support utility systems first have been qualified and, second, have been qualified within the specified process ranges prior to executing the process validation manufacturing runs.
The lack of equipment or utility system qualification is a common gap discovered during inspections and has often caused entire process validation efforts to be disregarded. In addition (and perhaps more critically), many unforeseen commercial production problems may arise when these activities have not been completed prior to process validation production runs.
For example, a contact lens facility uses a compressed air utility system. The system is designed to deliver a specified quantity of air to actuate automatic equipment (e.g., control valves). However, the manufacturing equipment and processes were modified over several years without the compressed air system being qualified.
During process validation, lenses were found to be out of specification. It was determined that this was caused by an inconsistent amount of monomer being dispensed (a raw material often used in contact lens manufacturing). The manufacturer should have performed system capacity testing, which is a common test performed in qualifying compressed air systems. If utility system qualification had been verified as a prerequisite, the problem would have been avoided.
Manufacturing and Inspection Instrument Calibration Verification. During prerequisite efforts, verify and document that each instrument used in the manufacturing and testing process that requires periodic calibration is within the current calibration interval and remains within that interval throughout the intended time frame of activity. Doing so reduces the risk of failures and rework during process validation.
For example, a validation engineer managed a shipping validation project for a temperature-sensitive product using numerous rented temperature and humidity monitors. When the data were collected and reviewed, it was noted that several of the instruments had results just out of the specified ranges. Upon investigation, it was noted that numerous instruments used in the study went out of calibration during the process, resulting in questionable results. All product shipped was then considered of questionable quality, as was the study, itself requiring a redo of the process and lost saleable product.
In addition, be sure to assign calibration points to bracket the intended ranges. Both process validation and calibration efforts often seem to overlook this key regulatory step.
Component Status Verification. Just as the manufacturing equipment and utilities must be able to perform within predetermined criteria, the components that go into the product must also meet predetermined specifications. As dictated by the QSR, a component must be tested and approved prior to use. The acceptance of the components called for in a process validation should also be verified prior to use. This may seem to be a redundant task, but spot-checking this aspect of the quality system prior to a critical effort such as process validation makes good business sense, while also being a specific regulatory requirement.
Consider the situation in which a contract manufacturer received a purchase order for an adhesive. Of course this activity requires process validation, for which both parties agreed to the minimum of three consecutive batches for process validation. The adhesive lots were assigned to be used in the three process validation batches.
As typically is the case with contract manufacturing, the time period for manufacturing each lot was dictated by the customer's order of the product. Due to an unanticipated lack in the customer's product sales, the third batch was manufactured more than a year after the first two validation batches were made. The shelf life of the active ingredient was only one year and had therefore expired. However, no status prerequisite check was performed prior to manufacture. Upon testing of the third lot, the quality control testing laboratory found the adhesive to be subpotent.
An extensive investigation was conducted, which resulted in the batch failing and all three consecutive batches for process validation having to be redone at the manufacturer's expense. This situation could have been avoided with a simple verification of raw-material status prior to manufacture of each process validation batch.
Specified Process Parameters Verification. If a product has been thoroughly developed, all of the critical manufacturing process parameters (i.e., processing ranges) that are specified in the DMR are based on results obtained during the process development effort. Parameters are then verified during the confirmation run or technology transfer phase.
However, many times one or more ranges specified in a DMR are not associated with any justification at all (i.e., where the range came from in the first place). Although it may seem to be a time saver to simply run the process validation with specified yet unsubstantiated ranges (versus generating a development report retrospectively), doing so truly presents a significant risk.
Although it should have been done earlier, ranges can be challenged or assessed during the process validation effort. Be warned, however, that waiting until this step of process validation carries a significant cost risk if a failure occurs during execution of the runs. Of course, even if the process is well characterized and well established, it is at risk for failure.
Without some documentation supporting the range specified (e.g., a development report), a processing failure associated with a specified process parameter can only be assigned a defendable corrective and preventive action if it involves a thorough retrospective analysis of a statistically significant number of historical batches for which the specified process parameter data are obtainable.
Of course, the very lack of documentation might lead a savvy auditor to question the development of parameters for other products as well. As you can see, not having specified process parameters can be very costly on many fronts. The only way to avoid such a situation is to verify and document the origin of each specified process parameter in DMRs before beginning process validation runs.
Conclusion
By using the process validation prerequisite approach, many of the potential pitfalls and hazards along the process validation route can be avoided before costly production runs.
Not only does this approach make good economic sense, but it can also demonstrate, during government and customer audits, that quality is built into the process and that a quality systems approach to regulated product manufacturing is alive and well in the facility.
Nancy Cafmeyer is a consultant at Advanced Biomedical Consulting LLC (ABC; St. Petersburg, FL). She can be reached at [email protected]. Jonathan Lewis is a principal at ABC. He can be reached at [email protected].
Bibliography
Code of Federal Regulations, 21 CFR 211.
Code of Federal Regulations, 21 CFR 820.
Guidance for Industry, Q7A, Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients (Rockville, MD: FDA, August 2001).
“Process Validation Requirements for Drug Products and Active Pharmaceutical Ingredients Subject to Pre-Market Approval” in Compliance Policy Guide Manual (Rockville, MD: FDA, 2006), 7132c.08.
Shaw, Arthur, Guideline on General Principles of Process Validation (Rockville, MD: FDA, May 1987).
Copyright ©2008 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like