New FDA Indication for Medtronic CoreValve: It's a First
March 31, 2015
CoreValve becomes first transcatheter heart valve approved for valve-in-valve procedures.
Chris Newmarker
|
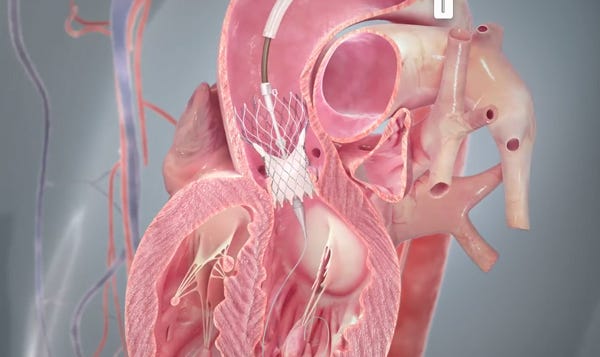
Rendering of the CoreValve right after its transcatheter deployment. (Image courtesy of Medtronic) |
FDA has cleared the way for Medtronic's CoreValve to be used in more patients in the United States.
The agency on Monday announced a new indication for CoreValve in which the device can be used in patients whose existing artificial heart valves are failing, but are considered too high risk for traditional open-heart surgery.
This is the first time that FDA has cleared a transcatheter heart valve for so-called "valve-in-valve" replacement, in which the valve is deployed inside a failing artificial heart valve.
"The approval is an important expansion of the authorized use of the transcatheter aortic valve replacement technology," William Maisel, MD, deputy center director for science and chief scientist in FDA's Center for Devices and Radiological Health, said in a news release.
FDA's decision could provide an extra advantage for CoreValve versus its major competitor in the United States: Edwards Lifesciences' Sapien valves.
The news of the VIV indication comes on the heels of the CoreValve receiving approval in Japan for use in patients with severe aortic stenosis who are unable to undergo surgery.
FDA approved the CoreValve System last year for patients at extreme or high risk for surgery. It received CE Mark for VIV procedures in May 2013. Since receiving its first CE Mark in 2007, it has been implanted in more than 75,000 patients in more than 65 countries.
CoreValve works for valve-in-valve procedures because of its supra-annular design meant to maximize blood flow, according to a statement Medtronic provided to Qmed.
Medtronic said: "Unlike other TAVR devices available that have an intra-annular valve, the CoreValve supra-annular design allows the artificial valve to be positioned above the failed surgical valve, which allows for a wider valve opening for blood to flow out of the heart.
Additionally, the self-expanding nature of the valve allows it to continue to provide pressure on the failed surgical valve, expanding it outwards, and thereby allowing it to create an essential seal that minimizes valve leakage."
The new indication comes after an Expanded Use Study, an observational arm of the CoreValve U.S. Pivotal Trial, found low rates of mortality and stroke: a combined rate of 4.2% at 30 days and 10.7% at six months, according to Medtronic.
Refresh your medical device industry knowledge at BIOMEDevice Boston, May 6-7, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like