December 1, 2016
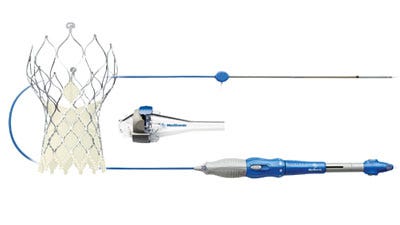
Medtronic has gained reimbursement approval for its CoreValve Evolut R transcatheter aortic valve in Japan and is now launching the device, making it the first recapturable TAVR system in the country.
|
The Japanese Ministry of Health, Labor, and Welfare (MHLW) just granted reimbursement approval for Medtronic's CoreValve Evolut R, and the company is launching the transcatheter aortic valve (TAVI) in the country. The launch will mean the TAVR device is the first recapturable system in Japan, according to a company press release.
The reimbursement approval and launch follows Shonin regulatory approval, which was granted in November. The technology is indicated for severe aortic stenosis (AS) patients who cannot undergo surgery. Such treatment decisions are determined by heart teams.
Medtronic's earlier-generation CoreValve System was approved in Japan in March 2015 and has been in use. This latest launch comes right on time, as Evolut R's entrance into the Japanese market was anticipated in the second half of the company's 2017 fiscal year (November 2016-April 2017).
"TAVI continues to grow as an established treatment for inoperable AS patients in Japan and we are excited to have a next-generation, self-expanding option, as the Evolut-R system has demonstrated exceptional clinical results in studies globally," Yoshiki Sawa, MD, profession in the Department of Cardiovascular Surgery at Osaka University Graduate School of Medicine in Osaka, Japan, said in the release. "Clinical data show the advancement of recapturability with Evolut R gives physicians more confidence during the procedure, providing different advantages not currently available in other TAVI systems."
The CoreValve Evolut R TAVI system is self-expanding with a delivery sheath profile of 14 Fr, the lowest on the market, according to Medtronic.
Data presented at the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting in early November showed a 91.4% survival rate and 5.1% disabling stroke rate in 241 patients implanted with CoreValve Evolut R as part of the U.S. IDE study. The rate of moderate paravalvular leak (PVL) was 3.7% while there were no cases of severe PVL. The permanent pacemaker implantation rate was 18.3% at one year.
"This approval stems from our global commitment to building a market-leading innovation pipeline in the transcatheter space, and we look forward to supporting Japanese heart teams as they look to next-generation technologies to improve valve performance for a broad range of patients," Rhonda Robb, vice president and general manager of Medtronic's Heart Valve Therapies business, said in the release.
Medtronic continues to advance its TAVR portfolio. At Medtronic's Cardiac and Vascular Group (CVG) analyst briefing during the TCT conference on October 30, Michael Coyle, CVG group president briefly outlined the timeline for the next-generation product, Evolut Pro. "Our next-generation Evolut R, the Evolut Pro, which adds the skirt technology to address the mild and moderate PVL rates that are already very low in the existing product, will be coming to market. We would expect [this] in the first half of our next fiscal year."
Coyle was referring to launches in Europe and the United States. The company's next fiscal year begins at the end of April 2017.
[Image courtesy of MEDTRONIC]
About the Author(s)
You May Also Like