Medtronic is shelling out $100 million to buy a company that makes a mesh cover to more effectively capture and retrieve clots in patients who have suffered an acute ischemic stroke.
September 28, 2015

Medtronic announced Monday that it has bought Lazarus Effect, a Campbell, California company that has developed products to treat ischemic strokes for $100 million in cash.
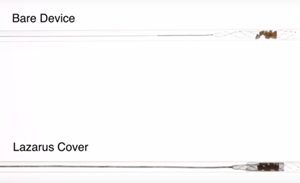
Lazarus Effect has a "mesh cover" technology that helps in the capture and removal of clots. The product is a nitinol "mesh cover" that folds over a stent retriever device during clot retrieval and essentially traps the stent with the clot inside similar to the way candy is contained in a candy wrapper. The product is complementary to Medtronic's Solitaire stent retrieval platform.
New guidelines from the American Heart Association and American Stroke Association encourages the use of endovascular treatment for acute ischemic strokes in selected patient for the clinical benefit they offer.
|
The Lazarus mesh cover is intended to be more effecting in capturing and removing clots than a bare stent retriever. |
The standard method of treating acute ischemic strokes is to quickly administer a clot busting drug intravenously. Now clinical studies are showing that adding a device component such as a stent retriever device provides more benefits than medical therapy alone. The Lazarus device is meant to be used as a covering for the stent retriever device to make its use even more effective.
"Medtronic has been a significant supporter of the recent clinical work showing improved outcomes of ischemic stroke patients treated with endovascular therapy," said Martin Dieck, Co-Founder, President & CEO, Lazarus Effect, in a Medtronic news release. "Their support of data driven clinical evidence and the success with their SolitaireTM stent retriever device make them the clear market leader for treating ischemic stroke. Lazarus Effect is pleased to bring our innovative technologies together with Medtronic's market leading therapies."
The Lazarus Cover device receieved CE Mark in Europe in November 2014. It is not cleared for sale in the U.S. Medtronic will integrate the acquisition into its Neurovascular business under the umbrella of the Restorative Therapies Group.
Arundhati Parmar is senior editor at MD+DI. Reach her at [email protected] and on Twitter @aparmarbb
To learn more about medical devices and trends in the marketplace, attend the two-day MD&M Minneapolis conference, Nov. 4 and 5 at the Minneapolis Convention Center. |
You May Also Like