Medtech Startup Showdown 2016: Round 2—Noninvasix vs. NERv Technology
Noninvasixvs.NERv Technology
April 4, 2016
| vs. | ||
|
|
|
|
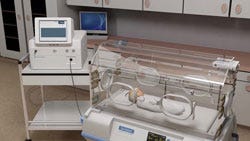
Describe your device and how it will benefit healthcare. | Each year, 270,000 preterm and low-birth-weight babies are born into a life of brain impairment due to a lack of blood flow to the brain after birth. Called hypoxic ischemic encephalopathy, this condition is a precursor to cerebral palsy and is responsible for 23% of all neonatal deaths and costs an average of $28,000 per patient. There is currently no technique for easily, repeatedly, and noninvasively monitoring or measuring cerebral circulatory adequacy. Using patented optoacoustic technology, Noninvasix is developing a monitoring system for at-risk babies in the NICU to measure the amount of oxygen in the brain in real time. Using a sensitive, wide-band head strap to access the superior sagittal sinus through the front and back fontanelles of the baby's still-forming skull, Noninvasix's optoacoustic monitoring system sends short laser pulses of near-infrared light into the brain. Hemoglobin in the blood absorbs the light at different frequencies depending on whether or not it is carrying oxygen. Absorption causes rapid thermal expansion of the hemoglobin resulting in a measureable acoustic wave whose amplitude is proportional to the concentration of hemoglobin. An acoustic transducer then detects these waves, and time resolution of the resulting signal determines the depth from which the signal was derived. The high resolution of this optoacoustic technique has been shown in pre-clinical studies to yield real-time and continuous measurement of blood oxygenation. |
| NERv is developing an implantable biochip to monitor a patient's health after surgery. The biochip is capable of detecting post-operative complications in real-time. The biochip is designed to target surgeries in the abdominal region, such as operations in the urinary and digestive systems. The biochip is very small, provides instantaneous feedback, and is made entirely out of biocompatible materials, making it completely harmless to the body. The biochip's biosensors continuously gather data about the body and analyze them. If an unexpected change happens, the sensor analyzes the change and sends feedback to the physician. The data is sent to a receiver wirelessly. The receiver is located in the trans-dermal patch placed on the wound after surgery. The receiver then transmits the data to the physician in order to determine if a complication is about to happen. Concurrently, the receiver alerts the patient if a complication is detected in order to seek medical attention immediately. |
How does your product differ from the competition? | In contrast to other purely spectroscopic techniques, such as near-infrared spectroscopy, this technology provides an absolute, rather than relative measurement, and can be targeted to specific blood vessels, such as the sagittal sinus vein in the brain. Such capabilities allow optoacoustic oximeters to be more broadly applicable than pulse oximetry. Furthermore, all NIR spectroscopic techniques rely on returning or transmitted light; therefore, they have limited ability to separate the signal derived from venous saturation, which reflects tissue oxygen uptake, and arterial saturation, which represents a component of oxygen supply. Although NIRS techniques have provided vital information on neonatal cerebral circulation they have not yet proven useful for routine clinical monitoring. Real-time monitoring is not a reliable feature of the small catheters currently utilized in the neonatal and pediatric setting. |
| Our technology is a disruptive one and thus there are no real competitors in our field. The nearest form of technology that provides value similar to ours is the medical imaging technology currently on the market, such as CT scans, MRIs, and radiography. These imaging techniques require the patient to show signs of sickness, before they can be used to identify the type and location of complications. However, they still fail 30% of the time (according to the Canadian Association of Radiologists). Our technology is predictive, real-time, and more efficient than the current alternatives on the market, promising to decrease the cost of inpatient healthcare. |
Do you have customers yet? | A third-generation prototype has been safely tested in adult humans and neonates in Houston, TX. |
| Our product will be tested in animals within the next six months. NERv has created a unique business model, where it will be targeting the veterinary market in order to prove clinical and commercial value for its product, before beginning clinical testing in humans. |
How much money have you raised? | $2.2 million |
| NERv has raised more than $100,000 in nondilutive capital. NERv has also raised approximately $125,000 in in-kind services from institutions in Ontario and throughout Canada. |
Who are your investors? | University of Texas Medical Branch, the Texas Emerging Technology Fund, and private investors |
| -- |
What is the next milestone for your device? | A Series A round in the amount of $3.75 million is underway. Within 24 months of closing the Series A, Noninvasix expects to complete a production prototype, perform clinical trials and obtain FDA 510(k) clearance. The company submitted a presub to FDA in February 2016, with expectations of a decision on the appropriate clinical regulatory pathway in April. |
| The next milestone will be to begin testing the biochip in animals to prove clinical and commercial value. |
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
