Medtech Startup Showdown 2015: The Winner Is...
Which company beat out all other contenders to be named champion of our Medtech Startup Showdown?
April 14, 2015

The final round of our Medtech Startup Showdown drew a staggering response--14,387 people cast votes for the two finalists: Interrad Medical--SecurAcath and BioDirection Inc.
The results are in and the winner is...Interrad Medical--SecurAcath, at over 10,000 votes or 70.6% of the total.
Congratulations to Interrad Medical--SecurAcath and a big thank you to all our competitors and voters. Look for a profile of the winner in an upcoming issue of MD+DI.
| |
|
|
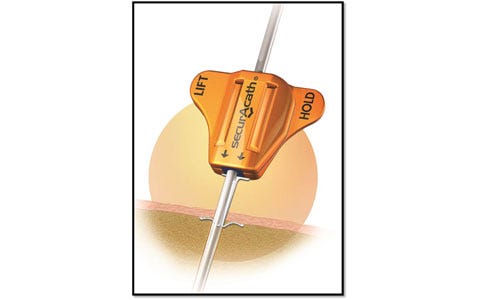
Describe your device and how it will benefit healthcare. | SecurAcath is a new method for catheter securement that does not require adhesives or sutures. The unique design of the SecurAcath secures right at the catheter insertion site using a small, blunt anchor that deploys in the subcutaneous tissue just beneath the skin. The device decreases the total cost of patient care by reducing complications associated with catheter securement. The SecurAcath dramatically reduces catheter migration and dislodgement, decreases catheter replacement costs, improves efficiency, and allows 360-degree site cleaning while secured. Improved stability combined with better site cleaning may reduce catheter-related infections. |
How does your product differ from the competition? | The SecurAcath subcutaneous catheter securement technology has many benefits compared with adhesive devices or sutures. SecurAcath offers a low catheter dislodgement rate, which dramatically decreases catheter replacement costs. It decreases the time required to secure, maintain, and remove catheters. The SecurAcath lasts the life of the catheter and, unlike all adhesive securement devices, does not need to be replaced at least weekly. The device design allows for improved catheter site cleaning and minimizes catheter movement, which may reduce catheter-related infections. SecurAcath is also sutureless, eliminating the potential for costly needle stick injuries that can occur when suturing catheters. |
Do you have customers yet? | The device has FDA and CE clearance and is being sold in the United States, Canada, and Europe.
|
How much money have you raised? | Approximately $25 million
|
Who are your investors? | Angel investors |
What is the next milestone for your device? | Sales revenue growth |
[Image courtesy of STUART MILES/FREEDIGITALPHOTOS.NET]
You May Also Like