Medtech Startup Showdown 2015: Round 1—Solaris Technology Group vs. Drug Free Therapeutix
Solaris Technology Groupvs.Drug Free Therapeutix (DFTx)
March 24, 2015
| vs. | ||
|
|
|
|
Describe your device and how it will benefit healthcare. | Solaris has developed a process for making 3-D, 100% open-cell materials that can be used as a standalone medical device or as a component in a device. It is pursuing a seven-day dressing with wound healing and scar reduction capabilities and a resorbable mesh for plastic and abdominal surgery in parallel. Benefits include improvement in performance and cost as compared with existing products, as well as improved biocompatibility, reduced capsule formation, and improved aesthetic appearance post surgery. Application areas include tissue engineering and scaffolding, tissue integration, fixation, wound healing, and scar reduction. |
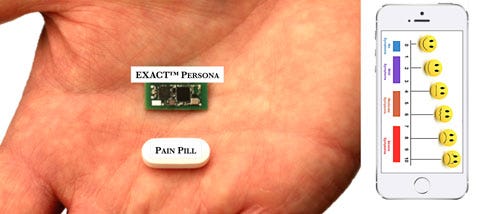
| The Exact device maximizes the quality and safety of therapy from existing FDA-approved neurostimulators for chronic pain by linking changes in patient-reported pain symptoms to the measured neural responses to electrical stimulation delivered by an implanted or external (e.g., TENS unit) stimulator. It then transforms the device programming interface from a complicated set of adjustable electrical stimulus characteristics to a set of dials that directly control the quality of pain relief, letting patients convey their experience to the stimulator and teaching it to meet their therapeutic needs. Most neurostimulators can be enhanced with Exact through a firmware or software upgrade. |
How does your product differ from the competition? | Three-dimensional, completely open-cell, fully biocompatible products are not available. Our product can be customized, whereas competitive solutions are limited by material choice and performance. Solaris offers benefits including implantability, superior mechanical properties, sterilization, particle count, abrasion, and cost. Open-cell polyurethane foam is the nearest competitor. The structure is great but the chemistry is poor for biomedical applications, and it offers poor biocompatibility for implantables. Particles, residual chemicals, toxic breakdown, abrasiveness, and tissue adhesion are also problems. Dermal products (cadaveric, porcine, bovine) are also available. These are animal-derived and come with high COGS and high price, and can cause allergic reactions. |
| Exact “learns” how to efficiently and effectively provide therapy based on signals measured from the patient's body and user-entered pain descriptors. An algorithm stores and analyzes the device settings, measured efficacy, and patient-reported efficacy to adapt to each user’s pain-relief needs. This simplifies programming, replacing a complicated stimulus parameter tuning system with a simple set of dials that control individual patient pain. Selling points include elimination of crossinterference, improvement in accuracy, improvement in signal-to-noise ratio, improvement in device robustness, reduction in sample requirements (enabling therapy to be initiated in minutes rather than months), and increase in market acceptance. |
Do you have customers yet? | Materials are being piloted with potential customers and investors. |
| We do not have customers yet, but are actively negotiating partnerships and licensing deals with 3 different companies. |
How much money have you raised? | $300K has been spent thus far in developing the process, obtaining samples across a wide array of materials, and securing intellectual property. |
| $100,000 |
Who are your investors? | The management team
|
| Friends and family |
What is the next milestone for your device? | Funding through one or more options: codevelopment of a specific product with a customer, partnering with a raw material or finished goods supplier, or an agreement with an angel or VC investor |
| Demonstrate the interoperability of our medical device software platform with third-party neurostimulators and fulfill regulatory requirements for our line of intelligent TENS units (Exact Persona) |


You May Also Like