Medtech Startup Showdown 2015: Round 1—Innoblative Designs Inc. vs. BioDirection Inc.
Innoblative Designs Inc. vs. BioDirection Inc.
March 23, 2015
| vs. | ||
|
|
|
|
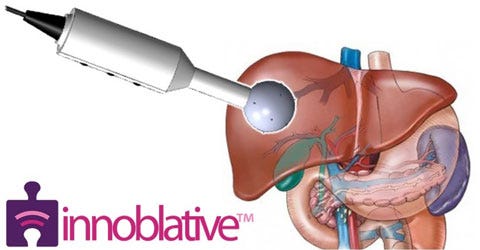
Describe your device and how it will benefit healthcare. | Innoblative Designs is dedicated to commercializing a radiofrequency ablation (RFA) technology that will innovate the treatment of cancer. Specifically, the device will allow surgeons to intraoperatively destroy residual cancer that may remain after the removal of a tumor using safe radiofrequency-generated heat, thus reducing or eliminating the need for reoperations and dangerous, lengthy, and expensive ionizing radiation therapy. Examples of such cases include liver cancers as well as early-stage breast cancers. Future platform indications being investigated include benign cysts found throughout the body. |
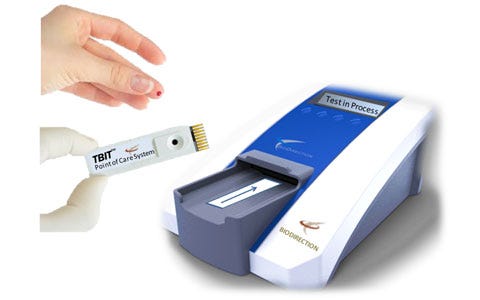
| The Tbit system is the first point-of-care platform for diagnosing concussions. This system uses a drop of blood to rapidly detect and measure abnormal quantitative levels of brain protein biomarkers released minutes after a concussion, or traumatic brain injury (TBI). Similar to a glucose meter, the low-cost Tbit system uses a drop of blood placed onto a disposable cartridge, which is then inserted into a portable analyzer. The device provides objective results in less than 90 seconds. BioDirection holds exclusive worldwide rights to over 14 issued patents, both domestic and international. |
How does your product differ from the competition? | Existing RFA applicators are designed for destroying primary, homogeneous solid tumors. Innoblative’s applicator is designed to destroy the heterogeneous tissue forming the walls of a complex-shaped cavity. |
| Today’s standard methods for concussion diagnosis rely on subjective, symptom-based tests some 30+ years old, the majority of which are paper-based questionnaires that have major confounding issues. Misdiagnosis, under-diagnosis, and even over-diagnosis of concussions is prevalent. In fact, even in the emergency room, 56% of patients who enter with a concussion are not diagnosed correctly or at all. The Tbit system is the world’s first simple point-of-care objective blood test for concussions. |
Do you have customers yet? | The company is currently prerevenue. Potential customers of the device, who have already expressed interest in serving as future clinical trial sites, include early academic centers (Northwestern University and Columbia University Medical Center). Future customers after the initial sites include regional providers (for example, Northshore University Health System and Allina Health) and community and rural hospitals. |
| The Tbit system is currently entering clinical testing in the United States. |
How much money have you raised? | More than $600,000 in funding from 11 accredited angel investors and nondilutive funding sources |
| Approximately $6 million |
Who are your investors? | Some of the company’s investors include: Harry Kraemer, former CEO and chairman of the board at Baxter; Bob Shaw, former CEO of Milex Products, a women’s health medical device and supplies company; and VentureWell, a higher education network that cultivates revolutionary ideas and promising inventions. |
| Primarily high net-worth individuals and one investment fund |
What is the next milestone for your device? | A preclinical animal study is planned at Northwestern University’s Center for Comparative Medicine in preparation for a 510(k) submission with an ablation of soft tissue indication for use. |
| Clinical trials |


You May Also Like