Edwards Lifesciences: A Passion for Patients
Putting patients at the center of its strategy has led Edwards to shake up the world of heart valve technology.
November 1, 2009
MANUFACTURER OF THE YEAR
|
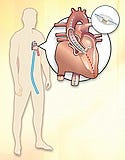
The SAPIEN valve is being evaluated in the treatment of patients with severe calcific aortic stenosis who are considered to be high risk for conventional open-heart valve replacement surgery. (Images courtesy of EDWARDS LIFESCIENCES (Irvine, CA)) |
Edwards Lifesciences appears to have recently had an overnight success with its foray into transcatheter valve technology. The technology has seen positive clinical outcomes during clinical studies and has been readily adopted throughout Europe and the Middle East, with several thousand heart valve replacements performed to date. The company has more than doubled the number of centers performing the procedure in the last three quarters from 70 to more than 145 sites.
Its exploration of how to replace or repair heart valves without open-heart surgery started 10 years ago when the company took a chance on a new—possibly disruptive—technology. That journey led to a new option for patients: a much shorter procedure instead of a laborious 6–8-hour open-heart valve replacement surgery. In other words, a short hospital stay and recovery instead of a week in the hospital and months of recovery time.
The company's transcatheter valve technology enables patients to have heart valves replaced while the heart is still beating. Many aortic stenosis patients are older and too frail for open-heart surgery. Without a less-invasive procedure, their options are limited to a compromised lifestyle, and often, death. “The approach we've taken is to try and stay modest in what groups of patients we treat so that we're really treating the right patients—the ones that are appropriate for care—and to be very rigorous in the collection of data.”
“This idea of potentially being able to do something to replace or repair a heart valve without open-heart surgery got our attention,” says Michael A. Mussallem, chairman and CEO of Edwards Lifesciences Corp. (Irvine, CA). “We started with a small R&D project. It was very much a transformational idea and, if you will, a disruptive technology.”
Mussallem says that when Edwards spoke to its existing customers, primarily cardiac surgeons, early on, they were understandably dismissive of the idea. At the time, he says, “it was not really comprehensible that you'd be able to replace a valve without surgery. Furthermore, when you don't stop the heart and you're able to do this with a small hole into the femoral artery or chest wall, it was remarkable.”
Mussallem recently attended TCT 2009, a symposium focused on transcatheter cardiovascular therapeutics for interventional cardiologists. “We were quite the story there. It wasn't very many years ago that we wouldn't have been a presence at all. There must have been close to 250 papers on transcatheter valves at that conference.”
When Edwards spun out of Baxter in 2000, Mussallem says that the company focused on its desire to be a faster growing company than it had been under Baxter. “We looked far and wide for those exciting growth areas that we could go into.” What he found, he says, was that “growth opportunities are very close to home for us.” Close to home for Edwards are technologies that address what Mussallem calls structural heart disease, in which heart valves are the largest component. “This has been a great opportunity for us. We're investing heavily in this space. My guess is that we're investing more than any other company by far.”
|
The minimally invasive transcatheter aortic heart valve replacement can be performed in either of two ways: the transfemoral approach (blue) in which the valve is inserted into the femoral artery or the transapical approach (red), via an incision through the ribs. |
It's in the DNA
Edwards was founded a little more than 50 years ago on heart valves. Miles “Lowell” Edwards teamed up with a surgeon named Albert Starr, and they commercialized the first heart valve. “We have been committed to heart valves ever since,” says Mussallem. “Along the way, we have been pioneers in tissue valves, which are the market-leading valves today, and pioneers in the repair of mitral valves.” And so, he says, the move into transcatheter heart valves was a natural one: “it's just in our DNA.”
Even as the company began its own transcatheter heart valve development program, it wanted to find ways to develop the technology faster—and first. It acquired a start-up, Percutaneous Valve Technologies (PVT), in 2004, and it was a decision that paid off. “We decided that if there was going to be a transformation in heart valves, that [we must do it]. We were willing to take a risk that others weren't willing to take at an early stage. In addition to our own program, PVT became the foundation of what we are commercializing today.” Mussallem says that it might appear like an overnight success to some people, but that it has been a long and bumpy road. However, he says, “It's one that we've always had confidence that there was something very meaningful here if we could solve some of the tough problems along the way.”
|
Employees at Edwards Lifesciences hand sew heart valves. |
Edwards SAPIEN transcatheter heart valve is currently being marketed in Europe and elsewhere in the international market. Mussallem says the company expects to the valve to bring in more than $100 million this year. At the same time, the SAPIEN is also going through a large-scale clinical trial in the United States called PARTNER.
“We're in the process of developing the next-generation product called SAPIEN XT and expect to introduce that product in Europe in Q1 2010. At the same time, we have even further developments in transcatheter heart valves in both the aortic position and other valve positions.”
Mussallem says he is fully aware that Edwards is introducing a technology into the company's portfolio that has the potential to be disruptive to its existing product lines over time. He refers to the term disruptive innovation as it was coined by Harvard professor Clayton Christensen, who theorizes that it's very difficult, if not impossible, for a market leader to introduce a disruptive technology, particularly one that might make its own products obsolete. “We decided we were not going to be victims to that kind of thinking—that we were going to embrace it and try to advance it, fully knowing that there was a real risk.”
|
An Edwards employee sews a Perimount pericardial tissue valve. It take 6–8 hours to finish a single valve, depending on experience. |
Edwards is focusing on two different approaches for the delivery of the valve into the body. One approach is to enter via the upper thigh and come up the femoral artery. It's the same route that is used for angioplasty or to deliver a coronary stent. The second method—the transapical approach—was developed after Edwards received some feedback from surgeons. They expressed interest in a shorter system that would enable them to insert the valve between the ribs and right up the apex of the heart. Moreover, they thought it would enable them to place it more accurately with fewer complications than by coming up through the femoral artery. Sales are split pretty evenly between the two approaches.
What's interesting, Mussallem says, is the way Edwards has approached the move into the transcatheter valve space as well as how the regulators have guided the company regarding the patient population that would benefit most from the technology. The patient group that Edwards has begun treating first consists of patients that are determined to be at high risk to undergo open-heart surgery—people, he says, who “don't have great options.”
Of course, the company's existing products continue to do well. “We still have a very robust business, and we strongly encourage patients that can have open-heart surgery to do so because it very much improves their quality of life—and the length of their life,” he says.
“There's such a great track record today for heart valve surgery for patients. Patients for the most part can feel comfortable that they're not going to need another surgery for 15–20 years or more. That still becomes the vehicle of choice until we demonstrate that we not only have a procedure that works in the near term but also that has a longevity. And that will take more time to prove that with data.”
Getting Down to Business
Part of its strategy has been to divest many product lines that were not really central to its valve and critical care monitoring businesses while increasing its investment in core areas. Key to this strategy is a major investment—13% of sales—dedicated primarily to structural heart disease R&D.
|
Michael Mussallem, chairman and CEO of Edwards Lifesciences, says Edwards is taking a “long-run approach” to business. |
To fulfill that goal, Edwards substantially increased its investment in R&D. When it spun out from Baxter, it didn't have much of a pipeline. Mussallem estimates that at that point, the company was spending 5–6% of sales on R&D. This year the company will spend more than 13% of its sales on R&D. “That's allowed us to build a pipeline of exciting products. And that pipeline is what has provided growth. Along the way, we've made a number of divestitures—literally divesting hundreds of millions of dollars of sales.”
With many companies restructuring and streamlining operations just to maintain profits during the downward spiral of the economy, Edwards has seen exceptional growth. According to the company, it demonstrated outstanding performance for 2008, including total underlying sales growth of 12%, making it the company's best year ever for top-line growth. These strong results led to an improved gross profit margin, as well as a 20% increase in earnings per share (EPS) compared with 2007. The company expects to carry last year's momentum into 2009 and to deliver strong results while making substantial investments in its future. It anticipates sales growth of 10–12% and EPS growth in the upper end of the 15–19% range.
“We really try to focus on those things that we're best at. I'm proud to say that more than 90% of what we sell are in number-one market positions,” says Mussallem. “We decided that the key to being able to drive growth and success for a company like ours was to be real innovators and to bring solutions forward for unmet patient needs.”
Mapping a Strategy
Mussallem says that for Edwards, the company's strategy is not about how big the company is, but rather about where the company is going. The company made a commitment to its investors, promising that while “transforming the company and building up that pipeline through innovation that we would deliver respectable mid-teens earnings growth along the way. We've been able to execute that. That's resulted in higher value.”
Edwards plans to sustain that level of investment in R&D. “My sense is that the level that we're at right now is pretty good for a company that really relies on innovation. We're fortunate, for example, that this year we'll grow our bottom line. Our last guidance was at the upper end of this 15–19% EPS range. If we can continue to do that, which is to drive strong earnings while we're taking up R&D—2008 was a very good year for us and 2009 was even better, so I expect us to be able to maintain those spending rates. As a matter of fact, if we look forward to what I expect to be an approval of the SAPIEN valve in the U.S. in 2011, I think our future will be bright.”
|
Edwards Lifesciences headquarters in Irvine, CA. |
Interestingly, Mussallem doesn't characterize Edwards as a very large company and rather considers it a mid-sized company. When most people think about the cardiovascular market, they first think about coronary artery disease and the treatment of that disease. The company's technologies, however, fall mostly into diseases where the structure of the heart needs to be addressed. “We try to bring best-in-class technology to that.”
Over time, he says, the company began to spend more time focusing on patients and less time focusing on specific products or technologies. When you focus on the patient, he says, it expands your horizons and allows you to go beyond boundaries that you typically think of. As an example, he says that in the past, the company might have just “stared at a heart valve and thought about how we could make that heart valve better.” Now, he says, “we find that we can have much more meaningful impact if we really examine the patient experience—and also the physician's experience. That's where we find opportunity.”
Edwards has had to shift its strategy slightly as its new technology meant a new customer—the interventional cardiologist. In the heart valve business, it had worked largely with cardiac surgeons. With transcatheter valves, those surgeons still play a substantial role, but Mussallem says that they now see a team approach with the cardiac surgeon and the interventional cardiologist together. “We've been adding resources to serve that specialty as well. Most of our resources so far have been clinical resources to help with the training and the support needed. Over time, that will continue to grow. Our strategy has not changed dramatically. We have a strategy that places the emphasis on clinical superiority.”
To achieve that, he says, it means “you need to innovate in a very thorough and responsible fashion. Our belief is that you're rewarded when you get that right.” He cautions that this strategy is not without risk. “It's a very competitive marketplace—there are some other very good companies out there that also provide solutions. We don't distinguish ourselves by having the biggest bundle or the lowest-priced product. We try and focus on clinical superiority. And what we do in transcatheter heart valves really fits that broader strategy.”
Clinical Trials
Mussallem characterizes the results of the clinical trials for the SAPIEN transcatheter valve “remarkable.” Having said that, he also says that there are many opportunities for improvement, and that the company is focused on understanding which patients benefit most from this technology. He says the company is “working aggressively” with clinicians to address those questions, such as developing a more-streamlined delivery system.
Edwards has nine clinical studies that are either complete or in progress in Europe and the United States. Mussallem says that the company is dedicated to being rigorous in terms of its scientific approach, making sure that the company reports all complications, with a high level of accuracy on the data. “I guess you'd call it a long-run approach—which is generating a lot of data and being transparent in that data. That way we position ourselves to make continued progress and really earn the trust of the clinicians and patients.”
With a new technology like transcatheter heart valves, there's a great desire to see data. “As the pioneer in the space, we have every incentive to have data out there. We dedicated pretty heavy investments to be able to make those data available.”
The AdvaMed Chairman
It's important to note that Mussallem has also spent the last year as chairman of the board of AdvaMed, putting him front and center of the healthcare reform debates. “I really love my job and this company, and I really care about our industry as well. It's so rewarding to us as a company and to me personally to play a role representing the industry.” He chuckles as he says that he had no idea what kind of a year 2009 was going to turn out to be. “It doesn't happen very often that the healthcare system in the United States gets reshaped. To be able to be at the table and to try to represent the importance of our industry's work is something that's been a tremendous learning experience for me personally.”
He says he has learned a lot from “wrestling with the mighty issues around healthcare.” And, he became acutely aware of the vastness of the healthcare industry. “The device industry was larger than life to me. You get in these larger discussions and find that [the device industry] is just 6% of healthcare spending and that there are so many other substantial players in this. I see us as the toolmakers for all those healthcare professionals, but it's really through their hands that the patients get the benefit of this wonderful technology.”
Bringing Life to Company Culture
Mussallem says the company's culture is critical to its success. “We have a credo that we don't just put up on the wall but that we really talk about. The last line is ‘helping patients is our life's work and life is now.'” He says Edwards puts a lot of energy into helping all of its employees understand how their work touches patients everyday. Edwards creates video patient stories in order to “bring to life somebody that's been touched by our products.” The company shares the videos with the employees. It lets the patients, who Mussallem says are very generous in their comments, thank the company and the employees for their hard work.
“We arrange to have patients come to Edwards and meet the sewers and the inspectors of their valves. The impact that has on our employees is incredible. When you compare it to the speeches you might make to employees about ‘boy you should always focus on quality,' compared with someone saying, ‘I had a chance to watch my granddaughter go down the aisle,' it's extraordinary.”
Sidebars: Edwards Employee Engagement
|
The company surveys its employees every other year to make sure that it is “an employer of choice,” and it gets extraordinarily high scores (see the sidebar “Edwards Employee Engagement”). Because the company places a serial number on each valve, Mussallem says it enables the company to identify the person and the team that was involved in making that heart valve.
In the company's most recent employee survey, employees gave high marks to the statement, “I believe in the values of Edwards Lifesciences.” The company's values are a key reason that Edwards employees are so highly engaged and that it has been able to enjoy such success, Mussallem explains.
“The whole idea of having a passion for patients is something that glues us together,” says Mussallem. “We keep working on the right things that way. When we're able to execute—and it does help that we don't try to overreach but stay close to those things that we're good at—that's when we can really make a difference. I'll put the passion for patients at the center of our success.”
Sherrie Conroy is editor-in chief of MD&DI.
Copyright ©2009 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like





.png?width=300&auto=webp&quality=80&disable=upscale)
