CMS Killed My $80M Venture-Backed Startup
Sonitus Medical raised $80 million from investors including Arboretum Ventures, Aberdare Ventures, and Medtronic, but a negative Medicare coverage decision shuttered the company.
April 2, 2015
Sonitus Medical raised $80 million from investors including Arboretum Ventures, Aberdare Ventures, and Medtronic, but a negative Medicare coverage decision shuttered the company.
Arundhati Parmar
.jpg?width=700&auto=webp&quality=80&disable=upscale)
It's not often that you hear that a startup that raised $80 million from well-known investors, is revenue generating and whose technology has been hailed by the Cleveland Clinic has shut down.
And yet that is the story of California startup Sonitus Medical.
It developed the SoundBite Hearing System system that it billed as the world's first "removable bone conduction hearing device that transmits sound via the teeth." FDA cleared the product for patients who suffer from one-sided deafness and conductive hearing loss in 2011.
But late last year, when the CMS declined to cover the FDA-cleared device—deeming it to be a hearing aid, which is not covered, and not a prosthetic as the company had hoped—Sonitus Medical was cut off at the knees. It ceased operations in mid-January, and is in the process of a liquidation sale.
"We were a full-blown commercial company, and at the peak we had 85 employees and a commercial team of 25 people," he says, with a lingering tone of disbelief. "In our first year of real commercialization we had $4.2 million in revenue and we were on track to do $8 [million] to $10 million this year, but we had to shut the company down." Sonitus Medical's founder and CEO, Amir Abolfathi, talked with MD+DI in mid-March describing the turn of events at the company he founded back in 2006 in San Mateo.
It's a dramatic turn of events, especially given what entrepreneurs like Abolfathi understand about the system.
"There are really three pillars that I was taught when I started my career, and I thought that if you impact one of these pillars you are going to get rewarded—make the product cheaper, make the product safer, make the product more effective," says Abolfathi in a phone interview. "We took a prevalent surgical treatment into the office where we reduced the cost by half and we significantly impact patient safety because there was no surgery involved and we made it more effective."
And yet Sonitus Medical failed.
Abolfathi lays the blame squarely at the feet of CMS for having made what he describes as an arbitrary decision in how the agency defines whether a device is a prosthetic.
"They arbitrarily draw a line saying, 'No, you are not qualified for coverage because the way we draw a line between what’s a prosthetic and what’s a hearing aid is whether it involves surgery or not,'" he declares.
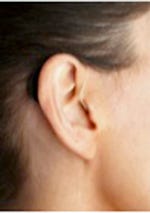
|
The SoundBite microphone worn in the ear. |
The SoundBite Hearing System is a removable device that is custom-fitted to a person's teeth. Like surgical bone conduction devices implanted on the skull, Sonitus Medical's product addresses the same patient population and has the exact same indication, Abolfathi says.
With SoundBite, a patient with one-sided deafness would wear the microphone in the ear and the mouthpiece on the teeth during the day. Before sleeping, the patient would take off the mouthpiece and the microphone and place it on a Sonitus charger.
The saga began in 2012 when Sonitus Medical decided that it would request CMS for a new Healthcare Common Procedure Coding System (HCPCS) code based on its consultants' advice. In November 2012, Abolfathi and the company's scientific advisors met with CMS officials to talk about the technology. A few weeks later, a CMS benefit determination letter arrived stating that the SoundBite Hearing System is a hearing aid and not a prosthetic, and so won't be covered.
"That letter really surprised us," Abolfathi recalls.
Thereafter, company executives and some independent physicians who were neither shareholders of the company nor consultatns, met with senior CMS officials to explain the benefits of the device. There were several meetings.
"We went to the Office of the Deputy Director and the chief medical director of CMS ... and some of our practicing physicians—some of whom were independent—wanted to make sure that CMS leadership was aware of how the technology worked and the similarities and differences between the existing surgical technology...," he says.
|
A patient wearing the SoundBite mouth piece |
Roughly around December 2013, CMS sent a letter informing Sonitus Medical that its formal request had been reevaluated, and the agency wanted to go through a formal rulemaking process in 2014 to update its coverage policy on hearing prosthetics.
"They did not say that we are going to cover you or anything like that," Abolfathi explains. "They said that they have reevaluated the situation and in order to [make a] final determination, they need to go through a rule making process...."
And then CMS dropped a bombshell on the hearing devices industry in July of last year.
It basically proposed a rule saying it wouldn't cover auditory osseointegrated implants—the predicate devices that Sonitus Medical's SoundBite was intended to be an alternative for—in the future.
An uproar ensued, forcing CMS to back away from dropping coverage altogether late last year.
But the status of SoundBite Hearing System did not change.
In November, CMS's final ruling was that it is not a prosthetic device because it is nonsurgical and is a hearing aid.
Some national payers were reimbursing Sonitus's system on a case-by-case basis and had said that they would amend their coverage if CMS issued a positive coverage decision.
"When CMS came with their final ruling, we just knew it was going to be an impossible task to get the other commercial payers to agree that we are a prosthetic," Abolfathi says. "Before [only] about 40% of our claims got paid based on individual policy's coverage for prosthetics."
The decision shocked Abolfathi and the company's investors—they were Medtronic and venture capital firms like Aberdare Ventures, Arboretum Ventures, Novartis BioVentures, Affinity Capital, European investor Abingworth, and In-Q-Tel, a not-for-profit investment firm that invests in technology to help intelligence agencies like the CIA.
"The CMS decision is ridiculous. It is not a transparent process" Abolfathi charges.
A spokesman for CMS did not address Abolfathi's accusation per se but provided, via email, an excerpt from the Medicare Benefit Policy Manual that highlights general exclusions from coverage, which include hearing aids:
Hearing aids are amplifying devices that compensate for impaired hearing. Hearing aids include air conduction devices that provide acoustic energy to the cochlea via stimulation of the tympanic membrane with amplified sound. They also include bone conduction devices that provide mechanical energy to the cochlea via stimulation of the scalp with amplified mechanical vibration or by direct contact with the tympanic membrane or middle ear ossicles.
Prosthetic devices that are covered are cochlear implants, auditory brainstem implants and osseointegrated implants, according to that excerpt.
But deeming the SoundBite Hearing System a hearing aid according to the definition above is incorrect, Abolfathi argues.
"This device does not work as a hearing aid - it's not designed to amplify the sound. If you put the device on the ear, it will not work," he says.
A reimbursement expert with no specific knowledge of Sonitus Medical and CMS's coverage discussion described this as a situation that was impossible or very difficult for the company to predict.
"That is an incredibly dramatic, unfortunate story, and what they got caught in was an unexpected new interpretation of that [statute] that just blew them out of the water," says Edward Black, president of St. Paul, Minnesota-based consulting firm Reimbursement Strategies. "I really can't think of another precedent where [a decision] impacted a company like this.
But Black underscores what is becoming increasingly clear in the current medtech landscape.
"FDA operates under the mantra of 'safe and effective' but not everything that is 'safe and effective' is considered 'reasonable and necessary' by Medicare for coverage," he says.
Black advises medtech companies developing new products to run reimbursement assessments at the same time as they are going through the FDA process.
In fact, paying more attention to reimbursement may not be such a bad thing.
"It’s really incumbent upon medtech companies to really watch those interpretations and not depend so much on FDA for predicate comparisions but look at what’s happening on the payment side," Black says. "I think we are going to see more tight interpretations of the statute."
Meanwhile, Abolfathi has been stung by the experience.
"Did we make any errors? It's a question that haunts me and my investors," he says. "My advice to other entrepreneurs is 'If you are going on a road, where there is reimbursement risk, don't go on that road. Go self-pay."
Arundhati Parmar is senior editor at MD+DI. Reach her at [email protected] and on Twitter @aparmarbb
[Feature photo courtesy of iStockphoto.com user heizfrosch]
|
You May Also Like