Building Acquisition Value through Patenting
A selling company should characterize its patent portfolio to show the potential of its technology and products.
May 1, 2008
MERGERS AND ACQUISITIONS
|
Illustration by iSTOCKPHOTO |
Early-stage funding for medical device firms typically comes from private investment. Although some companies may receive government subsidies, firms usually need venture capital (VC) as they start to build technology platforms and generate intellectual property (IP). VC investors are increasingly drawn to mergers and acquisitions as an exit strategy, because investors' returns are often elevated. Mergers and acquisitions also eliminate the underwriting commissions associated with IPOs and registration with the U.S. Securities and Exchange Commission (SEC) and its foreign counterparts. As a result, start-ups often focus on documenting value in anticipation of possible future negotiations.
In mergers, corporate valuation determines each party's slice of equity in the merged entity. In acquisitions, the acquired company must demonstrate value at least equal to its desired purchase price to satisfy investors. Although valuations of medical device companies vary based on the acquiring companies' criteria, the projected value of any acquired patent portfolio is critical to the terms of the purchase. Acquiring companies value patents based on the strength of their claims and their ability to materialize into revenue-generating products. Therefore, accurate valuation entails identifying the relevant market, competitors, and protected products, as well as how those products will generate revenues. This article discusses patent valuation as it relates to the medical device industry.
General Patent Valuation Methodologies
Valuation methods for patents can be crudely categorized as cost-, economic-, or market-based. However, many nuances exist within each category, and hybrid methods may be employed.
Cost-Based Methods. Cost-based methods are perhaps the most readily grasped by business executives because they are typically based on actual data that are documented and available. For example, a device firm's records of patent agents' or attorneys' fees associated with patent prosecution, as well as any fees paid to the applicable IP authority, will be available. Historical data on fee increases for the various IP authorities are also available. This information can be used to estimate the cost of prosecuting patent applications not yet granted and maintenance fees for granted patents. Unfortunately, cost-based methods do not account for economic gains attributable to patents, nor do they account for erosion of such gains by obsolescence or market-entry barriers. As a result, cost-based valuation is useful only in off-setting some of the value as determined by other methods.
Economic-Based Methods. Economic trends can be used to project patent values. For example, plotting trends between stock values and patenting by individual firms or industry segments can generally paint an accurate picture of portfolio values. Retrospective analyses of patent maintenance can indicate the continuing value of patents across a market and may be useful in estimating the time it will take a specific technology to become obsolete. However, a great deal of generalization is inherent in economic-based approaches; their usefulness depends on the force of the inferences drawn from a typically broad swath of data.
Market-Based Methods. Market-based methods generally involve tracking product revenues and life cycles in the relevant market and using that information to estimate the economic benefits that the portfolio will confer to its owner. Such methods require correct definitions of the relevant markets and accurate assessments of the competition before analyzing financial data. Financial data may be gathered from generalized market reports, market data subscription services from companies such as Hoover's Inc. or Frost & Sullivan, or SEC filings. In addition to measuring pricing and royalty rates in the relevant market, some data may be available on prices paid for comparable portfolios in similar transactions. Knowledge of historic market fluctuations and future market projections are also useful in market-based valuations. Revenue estimates must account for the time value of money as well as any associated risk.
Projecting Patent-Based Revenues
How a patent is used to generate business income depends on the owner's business model as well as the workings of the relevant market. Patents can protect revenues by blocking competition, or they can be used to garner licensing royalties. Royalties are sometimes paid by a willing licensee in exchange for the exclusive or nonexclusive right to make, use, or sell the invention within a specified product or geographic market. In other cases, royalties may be paid by a party solely to ward off a patent infringement suit that could result in payment of treble damages (three times the amount of actual or compensatory damages).
The medical device industry can be divided into companies that have the capacity to take innovations from concept to market, and those that cannot. Most start-ups cannot. They are typically built around innovators who develop technologies and then sell them to satisfy investors. The purchaser usually generates revenues from the technologies. Knowing the purchaser's postacquisition business objectives can help the seller understand the factors that are likely to be important to the purchaser's patent valuation. The seller should prepare to play up the portfolio's strengths in those areas.
If the acquiring company intends to sell products and limit competition with the patents, then the focus should be on demonstrating the force of the patent claims with regard to competitors. The patent's position within the U.S. Federal Trade Commission's (or its foreign counterpart's) view of the market is also crucial. Adopting a market definition that conflicts with the commission's position, for example, may result in greater acquisition costs. It may also cause the purchaser to flag some patents for possible divestiture and thus reduce the price it is willing to pay.
Any favorable decision rendered by one of the patent offices or the courts with respect to the scope or validity of patents' claims may be used to the seller's advantage, especially if similar questions come up during negotiations. If the purchaser is concerned with licensing revenues, royalty computations should be a major focus. If information on licensing-deal structures in the relevant market is available, it should be used as comparative information for substantiating value. If that information is scarce, there may be information on damage awards stemming from patent litigation within the market that include royalty computations.
Patent portfolios often contain one or more patent families—continuation, divisional, and foreign filings that protect the same core technology in different ways and markets. Continuations may contain small improvements over patent applications that are independently patentable (i.e., U.S. continuation-in-part applications). A divisional application may claim a surgical method, for example, if the parent application contains a device and a surgical method. In the United States, the U.S. Patent and Trademark Office may require separate applications for each claim. A foreign filing may contain identical claims and provide protection outside the country in which the original application was filed.
The seller should exploit the relationships between and among these filings, as well as the sheer number of them, to bolster the perceived value of the patent family as a whole. Common priority dates among document subsets should be identified in preparation for answering questions related to validity issues. The relationship of divisional applications is especially important in the United States, where the marketing of design-around devices by competitors may be prevented by surgical method claims, for example. The purchaser will often try to identify loopholes to drive its acquisition cost down, so the seller needs to identify those same loopholes and be prepared to demonstrate how they are filled.
Valuation of Medical Device Patents
The market-based approach to patent valuation is probably the most useful methodology for valuing medical device portfolios, because most companies base their technology platforms on unique solutions to existing problems in a defined patient market. The portfolio builds around that solution. Many innovators are, for example, entrepreneurial physicians who are intimately familiar with the target market before conceiving their inventions. In such cases, the target market is defined well in advance. Once the markets have been persuasively defined, the remaining valuation work may turn out to be a lot like painting by numbers: obtaining data on the size and competitive nature of the market, product revenues, reimbursement perspectives, and product life cycles.
Market data can be obtained from a variety of sources. This is especially the case when a device addresses a problem that is prevalent across a large market that crosses geographic borders and ethnic populations. For example, the worldwide prevalence of presbyopia may have enabled Acufocus Inc. (Irvine, CA) to easily gather information on the size of the patient market through publicly available news and academic and statistical literature. Acufocus developed corneal implants and associated surgical methods to treat presbyopia with a simple outpatient procedure, eliminating a patient's need for reading glasses or contact lenses.
In many cases, these resources can be used to complement a company's internal expertise. Acufocus was able to secure a successful equity investment with an option to purchase from Bausch & Lomb, which reported a target market of 50 million patients in the United States alone for the technology. Bausch & Lomb has the sales expertise to realize value in the eye-care market, but a persuasive market assessment may have offset the fact that Acufocus's patent portfolio consists primarily of applications not yet granted. When the relevant device market is small and procedures are risky and expensive, correct estimation of the market size may be elusive. In such cases, however, the patent landscape will likely be less populated, and thus the strength of the target patent claims will be easier to assess.
If the patent portfolio has already been commercialized to some extent, sales volume and pricing data should be accurately recorded and used to drive value. Many acquisitions, however, occur before commercialization. Indeed, commercialization is often the goal reserved for the prospective purchaser. In such cases, the seller should gather financial information on companies in the same space. This information is available publicly for some companies, and financials for private firms may be accessible to some extent through market research firms.
In any event, some inferences inevitably must be drawn. For example, one can infer that for a small company with minimal sales and marketing expertise, product revenues are more closely related to superior technology. By contrast, a large firm's product success may be influenced by a lengthy market presence and brand recognition. In some cases, overall product revenues may be reported in gross, and a full line of products must somehow be parsed to extrapolate a revenue estimate attributable to the technology. And it must be viewed within the same market as that protected by the patent portfolio.
Once the seller illustrates the device's market potential, it is critical to be able to chart the path to approval in each region of interest and the device's time from approval to obsolescence (the device's projected commercial life). Building a case for a large patient market and favorable reimbursement schemes is not that useful if the estimated commercial phase of the project is insufficient to garner returns on investment.
In the device industry, patent terms must be long enough to foreclose competition. Ideally, device firms recoup commercialization costs and make a profit during this time. The patent's useful life, defined as the length of the patent term that is likely to remain after FDA (or a foreign counterpart) has approved the patented device or component, must be determined in view of how quickly new technologies are likely to emerge in the relevant product market. The term remaining on each issued patent in the portfolio must be documented, including any term adjustments or terminal disclaimers. For applications that have not matured into patents at the time of the negotiations, the seller also needs to convince the buyer that patents will be granted, which requires evidence of other successful prosecutions and solid prosecution histories associated with any pending applications.
|
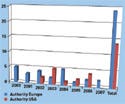
Figure 1. (click to enlarge) The cyclical distribution of a company's patents could strengthen its market position. |
A fairly recent transaction that illustrates some of these concepts is Integra LifeSciences's acquisition of IsoTis Orthobiologics. IsoTis has technology that reportedly eliminates a surgical procedure required to autograft tissue from a healthy area of the body to the site of a joint replacement for stimulating bone growth. The cyclical distribution of patents granted to IsoTis in the United States and Europe in the past seven years (see Figure 1) suggests continued innovation that should strengthen the company's market position. These patents may also enable the firm to capture a significant period of commercialization, making Integra's acquisition of the portfolio a desirable transaction.
International Considerations in Patent Valuation
|
Figure 2. (click to enlarge) Having patents and applications distributed among major regions worldwide may boost a firm's portfolio. |
Integra's acquisition of IsoTis also illustrates the effect of the international distribution of patents on the value of a company's portfolio. The distribution of the IsoTis patents and applications is well balanced across the Trilateral Cooperation of the United States, Europe, and Japan (see Figure 2). The fact that IsoTis is a Dutch company with significant European prosecution experience works in its favor due to the inability of most U.S. practitioners to prosecute in Europe. Many applications remain in the European portfolio that require continued procurement efforts. IsoTis may have been trying to drive its acquisition value up by offsetting the expense and uncertainty of European patent practice by a U.S. company. The same may be true for the Japanese efforts, which involve management of single-claim documents with lengthy pendency periods.
That European and world patent applications make up a large part of the IsoTis portfolio is also significant. Regional filings in Europe and international filings for U.S. companies are costly and require more effort than national filings. If the seller has regional European and international filings in process, the acquiring company may only have to select nations of interest and deal with the less-expensive and more-direct national filings in the selected regions. Therefore, a company with proper regional and international filings may use them to increase the purchase price when negotiating.
Conclusion
Establishing the valuation of a patent portfolio is a complex process that requires proper identification of the market share protected by the portfolio within each geographic region of interest. Procurement costs, competition, and regulatory barriers can drive values down. Therefore, the strength of the underlying innovations and the ability of the patent claims to protect the market share captured through those innovations must be highlighted.
Natural market forces such as design-around competition and obsolescence must be considered, and any information that downplays those concerns will be useful in substantiating value. There is no easy formula for negotiating a portfolio price, but companies should carefully consider all available information as it relates to the factors already discussed in this article.
Scott Lloyd is an analyst for Nerac Inc. (Tolland, CT) and a U.S. Patent and Trademark Office–registered patent attorney. He can be reached at [email protected].
Copyright ©2008 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
