Bos Sci Believes Synergy Warrants Pricing Premium
Boston Scientific executives think a pricing premium is justified for the recently FDA-approved Synergy Bioabsorbable Polymer Drug-Eluting Stent System and say they are excited for a post-approval study of the device. Here's why.
October 28, 2015
Marie Thibault
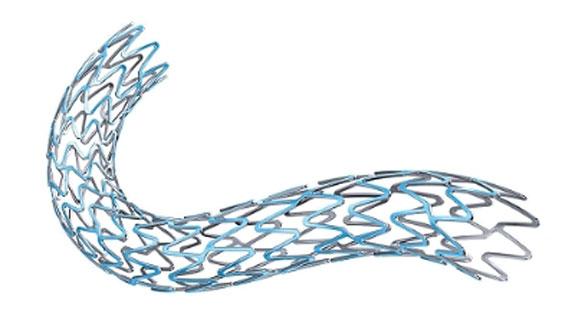
The Synergy Bioabsorbable Polymer Drug-Eluting Stent System received FDA approval in early October.
Boston Scientific executives think the company's Synergy Bioabsorbable Polymer Drug-Eluting Stent (DES) System is worth a higher price tag than its competitors. That may not seem like news at first—after all, most executives think their new products are worth paying for—but it wasn't long ago that analysts and market onlookers were calling the DES market commoditized and an area known for hospital pricing discounts.
The coronary DES market is still not an easy one, but for new offerings like Synergy, the first FDA-approved DES system with a bioabsorbable polymer and drug coating, as well as the fully-absorbable Absorb Bioresorbable Vascular Scaffold System from competitor Abbott (approved in dozens of international countries and awaiting FDA approval), pricing increases may be an option.
Still, some are skeptical of whether a pricing premium—depending of course on just how large that premium is—is warranted, even for new technology.
During Boston Scientific's October 28 third quarter earnings call, the management team pointed to a few reasons why they think a pricing premium is possible in the United States. The executives noted that in addition to the device's "unique benefits," the commercialization of Synergy in Europe included a premium price and the company's tiered pricing model allows them to offer a lower-priced DES as an alternative.
The size of the pricing premium will of course make a difference. According to a Seeking Alpha transcript of the earnings call, Glenn Novarro, analyst at RBC Capital Markets, said that survey work his team had conducted "suggests that you could probably get a 10% premium . . ." Management shied away from naming the exact premium they plan to charge, though back in July, on the second quarter earnings call, management noted that the pricing premium they use would not hurt adoption. Michael Mahoney, Boston Scientific president and CEO, said on the October 28 call:
We had that in the European market with SYNERGY priced at a premium. In the ten markets where SYNERGY's launched in Europe, it represents over 50% of our market share. And in the U.S., we also will have that same tiered portfolio with SYNERGY; the premium end, Promus PREMIER, which is the market-leading stent in the U.S., will be at a price discount versus SYNERGY. So we won't provide the specific premium that we're going to charge. I think it is important to note that this is a premium product, but as Dan indicated in his comments, this is a product that we will need to go through a contracting process with hospitals, given our pricing strategy. So we do anticipate excellent results in 2016, but we need to also work through that contracting process with hospitals . . . So, it's our job to prove the unique benefits of the platform that justify the premium. We've done that in Europe and that will be our plans as well in the U.S."
Rick Wise, analyst at Stifel, writes in an October 28 note following the earnings call that "the just US launched (and 1H2016 Japan) SYNERGY bioabsorbable polymer drug-eluting stent could drive not only favorable mix benefits, but also share gains, which should also contribute favorably to BSX's overall corporate margin profile."
Boston Scientific is also studying whether use of Synergy can enable a shorter course of dual anti-platelet therapy (DAPT). DAPT usually requires stent patients to take both aspirin and an antiplatelet agent for at least 12 months in order to reduce the risk of stent thrombosis. The company will start a post-approval study in the first quarter of 2016, the EVOLVE Short DAPT study, to evaluate safety of using DAPT for three months in patients at high risk for bleeding. Keith Dawkins, MD, global chief medical officer and executive vice president, pointed out on the October 28 call, "So we put ourselves in a position, if the trial is acceptable, of having a unique product in the U.S. in relation to a short DAPT, which we and the agency, the FDA, think is very relevant."
Want to catch up on the latest in medical device innovation? Attend Minnesota Medtech Week, November 4–5, 2015. |
Marie Thibault is the associate editor at MD+DI. Reach her at [email protected] and on Twitter @medtechmarie.
[Image courtesy of BOSTON SCIENTIFIC INC.]
About the Author(s)
You May Also Like


