Beware of These COVID Tests
FDA said 73,300 COVID tests made its way to market without authorization, and some of the tests even include statements that misrepresent the test as FDA-authorized.
February 18, 2022

As if we didn't have enough to worry about during the pandemic, some companies continue to take advantage of consumers by selling unauthorized COVID tests and other fraudulent COVID products.
The latest example of this behavior is the E25Bio COVID tests that were not only sold without FDA authorization, but actually pose a risk of injury during sample collection. The company was also brazen enough to indicate on the product labeling that it was an FDA-authorized test, according to FDA.
FDA said in a safety communication earlier this month, and again in a recall notice on Friday, that E25Bio sold its COVID-19 Direct Antigen Response Test directly to consumers without authorization and included instructions for collecting a sample from deep inside the nose, reaching the back of the throat (nasopharyngeal) or from the middle part of the throat (pharynx) just beyond the mouth (oropharyngeal). Self-collecting nasopharyngeal or oropharyngeal samples for SARS-CoV-2 testing could result in serious injury when this is not done by trained professionals, the agency warned.
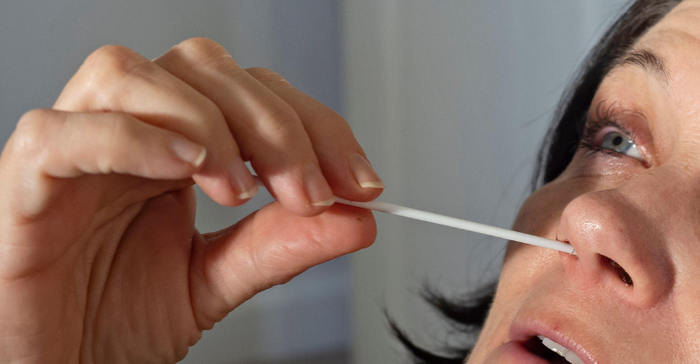
The company is now recalling the 73,300 COVID tests it distributed between September 2020 and November 2021. Because the test was not authorized, cleared, or approved by FDA, the agency said there is not sufficient data demonstrating the test's performance is accurate. This means there is a risk of both false-negative and false-positive test results.
The company's website claims that its COVID tests give results in under 20 minutes, cost a fraction of traditional testing, and are based on technology born out of MIT. Early on in the pandemic, the Cambridge, MA-based company even managed to get press coverage by mainstream media outlets such as USA Today, and the company's director of operations and communications was quoted about regulatory barriers to bring such testing to market.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
