9 Medical Technologies Poised to Explode in 2016: Endocardial Left Atrial Appendage Closure Devices
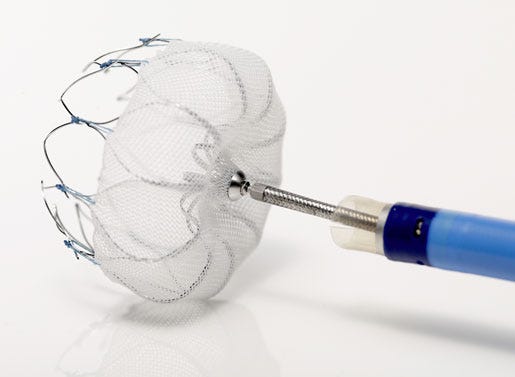
Endocardial Left Atrial Appendage Closure DevicesBoston Scientific's Watchman left atrial appendage closure device won FDA approval in 2015.Another technology we’re likely to hear a lot more about is endo
December 8, 2015
Endocardial Left Atrial Appendage Closure Devices | |
| |
Boston Scientific's Watchman left atrial appendage closure device won FDA approval in 2015. | |
Another technology we’re likely to hear a lot more about is endocardial left atrial appendage (LAA) closure devices—specifically Boston Scientific’s Watchman product, which won FDA approval this past March. This year saw what iData Research analyst Sean Collins calls a “controlled launch” of the product, with Boston Scientific focused on training doctors on how to use the device. “In 2016, when the doctors are trained and they’ve received positive results from the EVOLVE trials, I think there’s going to be strong growth and penetration wit Watchman devices.” With Watchman's main competitor, the Coherex WaveCrest LAA Occlusion System, not expected to gain approval in the United States in the next 12 months, Collins says Boston Scientific will likely have the market all to itself. | |
Continue on to "Top Medtech Companies of 2015" | |
| |
[image courtesy of BOSTON SCIENTIFIC] |
You May Also Like