2016 Medtech Company of the Year Finalists: Edwards Lifesciences
Edwards LifesciencesEdwards Lifesciences's could be first to market with a TMVR system.
October 19, 2016
Edwards Lifesciences |
Edwards Lifesciences could be first to market with a TMVR system. |
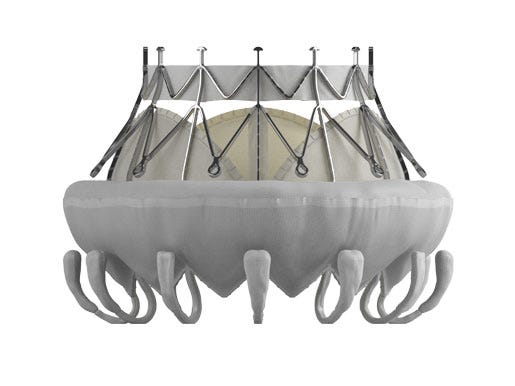
A perennial top name in the medical device industry, Edwards Lifesciences continued its solid performance this year. The Irvine, CA-based company has racked up repeated wins in its transcatheter aortic valve (TAVR) business over the past several months, including CE Mark and FDA approval for expanded use of the Sapien 3 valve in intermediate risk patients. Edwards is extending the technology's reach bit by bit; an IDE study of low-risk patients is ongoing, and Sapien 3 received regulatory approval in Japan in March. Meanwhile, its global transcatheter heart valve sales continue to grow by leaps and bounds, including a more than 40% year-over-year increase in the first half of 2016. The company's share price reflects these successes, climbing approximately 50% since January. With wins like these, Edwards seems likely to keep building its TAVR empire and bringing in a significant chunk of the total market opportunity, which is estimated to reach more than $5 billion by 2021. As if one blockbuster product category weren't enough, Edwards is also heading up the race for a commercial transcatheter mitral valve replacement (TMVR) system. TMVR therapy holds the promise of an even bigger patient population than TAVR, and several players are at work on devices. Edwards intends to be a pioneer in the field, just as it was first to market with TAVR. In July, about a year after Edwards acquired TMVR company CardiAQ Valve Technologies, Michael Mussallem, Edwards chairman and CEO, said the TMVR platform had since been enhanced and was poised to enter a CE Mark trial. There's no word yet on whether that trial has started, but you can bet clinicians, customers, and competitors care. |
[image courtesy of EDWARDS LIFESCIENCES] |
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
