10 Hot Devices We Can't Get in the U.S.—Endologix's Nellix
July 27, 2015
1 Min Read
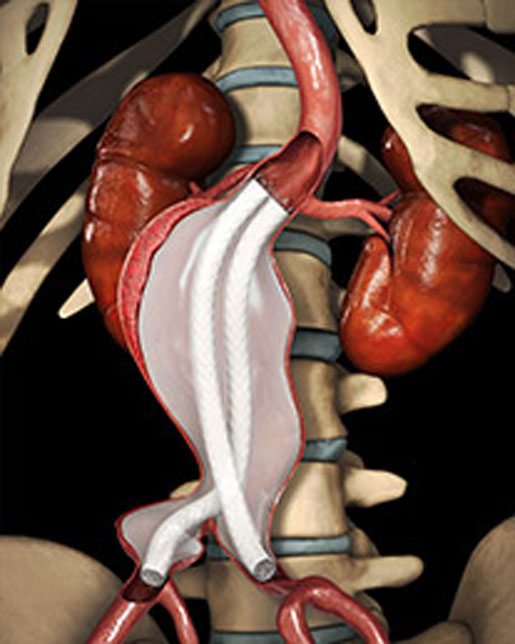
Endologix's Nellix
Endologix developed the Nellix endovascular aneurysm sealing system to give a minimally invasive option to patients who cannot receive conventional endovascular aneurysm repair (EVAR) for abdominal aortic aneurysms (AAAs). These patients may have challenging aortic anatomy that does not fit traditional EVAR devices. The Nellix system uses polymer-filled endobags to seal off the aortic aneurysm and prevent further aneurysm expansion or rupture. Nellix received CE Mark in January 2013 and has been much buzzed about at vascular medical meetings ever since. Analysts expect Nellix to gain FDA approval near the end of 2016. |
|
[Image courtesy of ENDOLOGIX, INC.]
Sign up for the QMED & MD+DI Daily newsletter.
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
