Boston Scientific Recalls 955 Guidewires after Patient Death
November 18, 2015
Boston Scientific is overseeing a Class I recall of the RotaWire Elite guidewire after being granted a PMA Supplement from FDA to change the contract manufacturer and the branding of the device. The device was sold to 30 hospitals as part of a "limited release."
Nancy Crotti
|
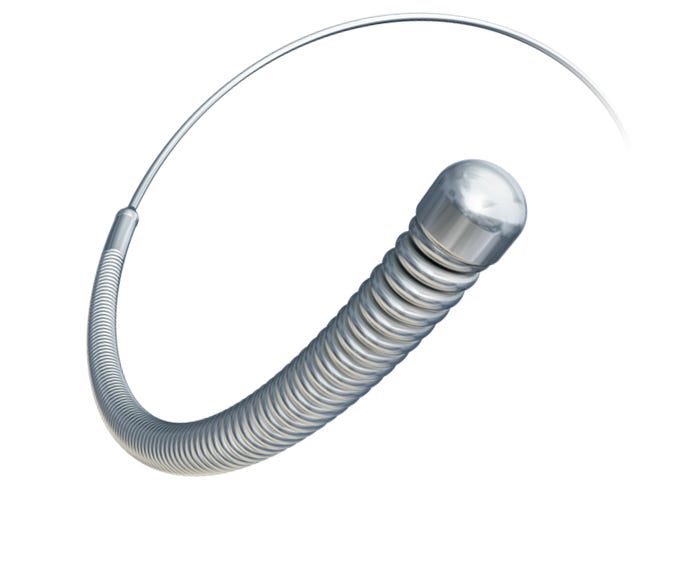
The RotaWire |
Boston Scientific has recalled 955 RotaWire Elite guidewires used to clear severe arterial blockages because the wires may break and cause patient injury or death. There were a total of three reports of the wires fracturing.
One patient died September 30, four days after the RotaWire Elite broke after a doctor threaded it through the patient's artery. The attached burr of the device perforated the vessel, according to the FDA adverse event report.
In the case of the death, the burr was spinning at 190,000-200,000 revolutions per minute at the time of the breakage, the adverse event report noted. The burr and part of the wire were removed, but 20-30 cm of wire remained in the patient, the report says. The patient was in Germany, according to a report by the Star Tribune of Minneapolis. Boston Scientific's Maple Grove, MN, office issued the recall.
The problems took place after June 19, 2015, when FDA granted the Marlborough, MA-based company a PMA supplement for "a core wire vendor change and the associated rebranding of the RotaWire Guidewire as the RotaWire Elite Guidewire." The agency granted the company permission to add "an alternate component supplier" for the RotaWire Elite. The adverse event report lists Boston Scientific - Costa Rica (Heredia) as the device manufacturer and the manufacture date as August 18, 2015.
Boston Scientific notified about 30 hospitals to which it had sold the device on a "limited release" basis about the voluntary recall in August, according to company spokesman Tom Keppeler. The recall does not apply to original RotaWire Guidewires, Keppeler added.
FDA classified it as a Class I recall and notified the public November 13. The agency's public notice says that it determined that a change in manufacturing practices led to the recall. Keppeler said he had no information about the wire manufacturer.
The product had been distributed in the United States as well as in Belgium, Germany, Great Britain, Italy, Japan, and South Africa.
Learn more about medical technology trends at BIOMEDevice San Jose, December 2-3. |
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like