February 9, 2016
Medicare coverage of the left atrial appendage closure device was an open question for Boston Sci going into this year. Now the question is resolved in the company's favor.
Chris Newmarker
|
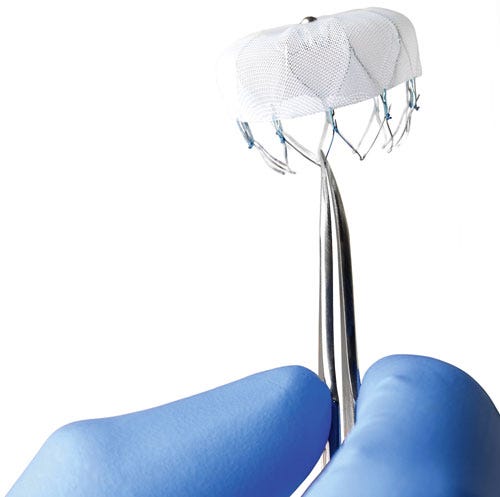
The Watchman's features include a self-expanding nitinol frame in five sizes, PET mesh fabric meant to promote endothelialization, and 10 fixation anchors to promote stability. (Image courtesy of Boston Sci) |
Boston Scientific stock shot up more than 5% in value during Tuesday morning trading after news that the company's Watchman left atrial appendage closure device would be covered by Medicare.
Approved a year ago by FDA, the Watchman has been ahead of schedule with its launch, according to Boston Scientific. However, the device--which prevents strokes by stopping blood clots in the heart from reaching the brain--faced limited Medicare coverage if a federal proposal went through in the U.S.The proposal, announced in November, would have made the Watchman ineligible for broad reimbursement because it was "not reasonable and necessary."
The final National Coverage Determination (NCD), released by CMS on Monday, was much better for Boston Sci, providing coverage under specific criteria, including that the patient is unable to take the long term oral anticoagulation that CMS considers to be the first line of therapy against stroke risk.
"We are very pleased CMS has established national coverage for this life-changing therapy for Medicare beneficiaries who have a reason to seek an alternative to long-term anticoagulation," Boston Scientific CEO Mike Mahoney said in a news release. "The final decision reflects more than a decade of robust clinical evidence and will facilitate additional data collection via a prospective national registry."
The CMS ruling is a big deal; Mahoney had previously estimated that the Watchman represents at least a $500 million worldwide market opportunity. Boston Scientific stock was up more than 5% in value, to nearly $17 per share, in Tuesday morning trading on the New York Stock Exchange.
Conditions under the NCD also include the person receiving the device to have a CHADS2 stroke risk number greater or equal to 2 if over 75, or greater or equal to 3 if over 65--along with suffering from various chronic conditions outlined in the NCD. An independent non-interventional physician must be involved with the decision-making.
The doctor performing the procedure to insert the Watchman must meet certain qualification standards outlined in the NCD, and both the patient and health provider must be enrolled in a prospective, national, audited registry.
The news about Medicare coverage for Watchman is especially good new for Boston Sci because its cardiac rhythm management products such as pacemakers and defibrillators have been a drag on sales growth. As is the same case with St. Jude Medical, Boston Scientific is behind its competitors when it comes to getting MRI-compatible devices approved in the U.S.
Along with Watchman, Mahoney has also been optimistic about other new products:
SpyGlass DS, launched in July 2015, is a single-use visualization system for the diagnosis and treatment of complex pancreas and bile duct disorders;
The Axios stent, used for transluminal drainage of pancreatic fluid collections, is also off to a "strong start;"
Boston Sci this quarter launched LithoVue, a disposable ureteroscope that provides visualization and navigation capabilities to diagnose and treat stones and other kidney, ureter, and bladder conditions;
In neuromodulation, Boston Scientific in the final months of 2015 launched Precision Novi, its first product of the primary cell non-rechargeable market.
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like