Aetna Mostly Halts Coverage of Once-Popular Hysterectomy Device
May 5, 2015
The move from one of the largest health insurers in the U.S. comes amid warnings from FDA and others that power morcellators can spread cancer.
Chris Newmarker
|
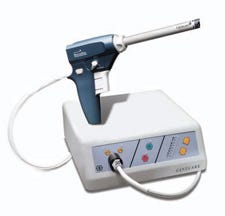
The Gynecare Morcellex Tissue Morcellator, as shown on Ethicon's website. |
Aetna in most cases will not pay for the use of power morcellators in myomectomies or hysterectomies, the Hartford, CT-based health insurer said Tuesday.
The new policy goes into effect May 15, an Aetna spokeswoman confirmed to Qmed. The decision comes amid FDA warnings that the spinning blades of the once-popular devices can spread unsuspected uterine sarcoma in the abdomen and pelvis.
Johnson & Johnson's Ethicon subsidiary already pulled its morcellators from the market last year, including the Gynecare Morcellex Tissue Morcellator, Morcellex Sigma Tissue Morcellator System and the Gynecare X-tract Tissue Morcellator.
The issues surrounding the morcellators have not only effected the companies that make them, such as Ethicon, but also companies such as Intuitive Surgical, which makes surgical robots for the early stages of hysterectomies, before morcellation takes place.
For its part, Minnetonka, MN-based UnitedHealth Group, the nation's largest health insurer, has placed new restrictions on its coverage of hysterectomies. But Aetna's move goes farther and is likely to influence other health insurers to do the same.
FDA now estimates that 1 in 350 women undergoing hysterectomy or myomectomy might have such an unsuspected uterine sarcoma, which is higher than previously estimated, Aetna spokeswoman Cynthia Michener noted via email.
When it comes to power morcellators, the "safety and efficacy of this approach has not been demonstrated," Michener said.
Doctors can request a clinical exception for Aetna coverage if the patient is pre-menopausal and wants to maintain her fertility and another surgical or non-surgical fibroid treatment wouldn't be effective. An exception is also possible if a patient has severe comorbidities, where the risk of death or severe morbidity of another procedure outweighs the risk of an undiagnosed sarcoma.
The Wall Street Journal first raised concerns about morcellators in late 2013. WSJ reported in March that doctors have already found other noninvasive ways to perform hysterectomies. Most are instead engaging in a "mini-laparotomy" that involves removing the uterus through a tiny incision above the pubic bone.
Refresh your medical device industry knowledge at MD&M East in New York City, June 9-11, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like