How 3-D Printing Is Changing Cardio and Ortho Applications
May 12, 2015
While 3-D printing is catching on, medical device manufacturers should consider it carefully before jumping in head first.
Bob Michaels
|
Materialise's patient-specific cranial implant printed using porous titanium. |
Several speakers will take the podium at MD&M East to offer their insights into 3-D printing. Among them will be Colleen Wivell, biomedical engineering manager at Materialise (Plymouth, MI), a provider of 3-D printing software and services. On June 11, Wivell will join a panel of experts whose objective will be to address the major speed bumps that medical device companies face in integrating 3-D printing into their manufacturing lifecycles.
"What's really unique about 3-D printing is that it adds material to build a part, as opposed to subtracting or molding a part," Wivell explains. "Thus, it is capable of producing really complex designs that can't be manufactured any other way."
3-D printing differs from other manufacturing methods in its build orientation. First, in 3-D printing operations, parts are built layer by layer. Second, because there are many different types of 3-D printing machines and materials, users must decide what works best for the products they manufacture. Third, print resolution varies depending on the combination of equipment and materials that a company chooses.
Despite these complexities, however, manufacturers may not always consider these factors when deciding to adopt 3-D printing in their operations. It would therefore be a misconception to think that a company can simply replace its manufacturing technologies with 3-D printing. "Just like the use of any other manufacturing technique, using 3-D printing requires a good knowledge base and expertise in order to create high-quality components," Wivell states. "Thus, manufacturers must understand what 3-D printing is all about and consider how they could benefit from it. As they begin to use the technology, they will come to understand its limitations and technical demands."
3-D Printing Applications
3-D printing is currently being used to build four types of devices: anatomical models, medical instruments, custom implants, and standard implants.
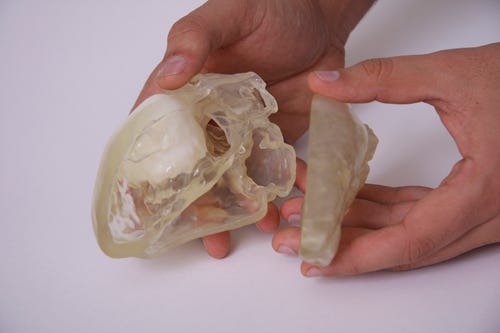
|
Example of a 3-D printed heart model highlighting a tumor. (Photo courtesy of Materialise.) |
Based on real patient data, 3-D printing is already being used to make anatomical parts. Several cardiovascular devices companies use anatomical models for flow-testing purposes in their R&D labs. For example, they may use such models in order to perform physical testing on new devices. Doctors, on the other hand, may use patient-specific models to practice surgical procedures. "These models," Wivell comments, "produce a great deal of value for clinicians and surgeons."
In the realm of custom instruments, the most popular 3-D printing application is the fabrication of orthopedic instruments, including cutting and drilling guides used to perform total knee replacements. But 3-D technology is also being used to produce instruments for total hip replacements, shoulder and ankle implants, complex wrist surgeries, and craniofacial reconstructions. Thus, the use of 3-D printing to produce medical instruments is growing in importance.
Custom implants is another, albeit smaller, area in which a patient's anatomy serves as the basis for designing a custom implant, Wivell remarks. It involves designing an implant for a specific patient because a standard part will not work. Some of the more common examples of custom implants include cranial reconstructions to restore defects in the skull and custom hip sockets to treat complex defects.
"For such custom applications, Materialise's Mimics Innovation Suite enables users to take CT, MRI, or other scan data to generate a 3-D model," Wivell says. "This model represents an actual person's anatomy. Engineers and doctors can then use it for 3-D printing, designing custom devices, or linking to finite element analysis and computational fluid dynamics."
Finally, 3-D printing is being used to produce standard implants. Some orthopedic manufacturers use it for low-volume applications in which it is cost-effective, while others print metal devices to take advantage of 3-D printing's ability to produce implants with rough surfaces or to produce devices with complex meshlike infrastructures to promote osseointegration.
Who's Who in 3-D Printing?
In the cardiovascular space, 3-D printing technology is finding widespread use for flow-testing purposes, according to Wivell. "For example, all the big players--the Medtronics and St. Judes of the world--are using 3-D printing to this end." Many large hospitals--including the Mayo Clinic, Boston Children's Hospital, Cedars Sinai in Los Angeles, and Cleveland Clinic--are also using the technology to produce patient-specific models. Meanwhile, in the orthopedic space, many large companies--such as Biomet, Zimmer, DePuy Synthes, Smith & Nephew, and DJO Global--are using 3-D printing to make surgical guides for a range of orthopedic procedures, including total knee, total hip, or shoulder replacements.
"If you look at the use of 3-D printing to produce custom implants, the list becomes smaller," Wivell says. "In this space, Materialise's daughter company Mobelife produces a metal implant that is used in complex hip surgeries for treating cancer patients or patients with congenital defects. So far, however, it is cleared for use only in the European market." Another daughter company, OBL, creates custom metal cranial plates using 3-D printing processes. In contrast, Oxford Performance Materials offers a custom 3-D printed biocompatible cranial plate made from PEKK.
Particularly noteworthy is a custom 3-D printed tracheal splint that was produced at the University of Michigan to treat a baby named Kaiba who was born with tracheomalacia. Normally, babies suffering from tracheomalacia are put on respirators until they outgrow the condition, but in Kaiba's case, this approach was insufficient for treating his collapsed airway.
Out of choices, Kaiba's family turned to the University of Michigan, where Glenn Green, associate professor of pediatric otolaryngology, and Scott Hollister, professor of biomedical engineering and associate professor of oral surgery, designed a custom splint for implantation on the outside of the baby's trachea. Made from a plastic material, the custom 3-D printed implant will resorb into the body in two to three years after the baby has outgrown the condition.
"Since Kaiba, the doctors have used this technology to treat other babies in the United States suffering from tracheomalacia," Wivell comments. "While it is currently not cleared for mainstream use, they are pursuing regulatory clearance. Nevertheless, this case has helped to elevate the use of 3-D printing and its applicability to medical device applications around the world."
Bob Michaels is senior technical editor at UBM Canon. Reach him at [email protected].
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)
