Better by Design: Seeking a Contract Molder’s Support Up Front
Ensuring quality in a part is the outcome of a carefully executed design process using all the expertise and analytical tools available through a contract molder. Here, design parameters are tested.
March 1, 2010
Medical device manufacturers begin the development of any product with a specific idea to meet a clinical need. However, having the idea is one thing; making it a reality as quickly and cost-effectively as possible is another. And, along with tight resources, many manufacturers may lack the broad expertise required from design to production to fully realize the potential of a new medical device.
Material selection, product testing, part and mold design (such as leveraging Mold flow, finite-element analysis (FEA), and other development tools), and advanced processing techniques are among the crucial areas of expertise a contract molder can contribute. Realizing such benefits to the fullest extent necessitates a shift in mindset for many OEMs and contract manufacturers. No longer can the parts of a supply chain function as distinct operations in traditional vendor-customer relationships. Instead, manufacturers must be more thoroughly integrated as one continuous value stream. A new level of commitment, partnership and, perhaps most importantly, trust is needed.
This article discusses those aspects of manufacturing and presents some examples of practical applications of working with a mold supplier.
Smart Material Selection
Material selection is vitally important to the success of any manufactured product. This is especially true for medical devices, which often carry demanding requirements for physical attributes such as antimicrobial properties, impact resistance, or extreme temperature capabilities. At the same time, there are increasing industry regulations such as eliminating the use of phthalates—a plastic additive that can enhance a material’s property but could be considered unsafe.
|
Engineering experience and proven processes translate a design concept into a solid model and then into a physical part. |
Determining the ideal material for any given medical device is a challenge. That’s why, early on in the design process, material analysis can be highly beneficial. It can help identify a high-performing or cost-effective material that fits the characteristics needed for the part.
OEMs need to ensure that partners clearly understand their requirements, as well as the product’s end-user application in the work environment. For example, say a contract molder is charged with producing a part for a handheld device that controls a medical instrument. Delving into the application further, the molder determines that the handheld device is subject to daily skin contact.
Over time, natural skin oils as well as petroleum-based products and lotions transferred from human hands can degrade common polycarbonate materials and increase the risk of breakage. The polycarbonate part might start out nice and shiny, but it would likely become brittle from this prolonged skin contact. Lab tests including UV chamber, chemical soak, and thermocycle testing would be able to spot this degradation problem.
The challenge then is to identify a more suitable material that isn’t as susceptible to degradation. In this case, the solution might be a polyester or nylon-based alloy. There are trade-offs with these materials, however, including diminished cosmetics (e.g., you can’t get that polycarbonate shine) and costs are higher.
Ultimately, the OEM, molder, and materials expert must work together to make the most appropriate material choice, based on the following:
The price of the material.
Ability to withstand the operating environment (temperature, chemical exposure, weatherability, etc.).
Product life expectancy.
Structural requirements.
Cosmetic requirements.
Consider another material used in some medical devices, polyphenylsulfone (PPSU), which is valued for its ability to withstand high temperatures. PPSU can be an excellent choice for a medical device; it’s often used for battery packs for pacemakers and dialysis equipment. But if the product in question requires sterilization, that poses a challenge to this material. PPSU could handle a high-temperature sterilization process, but in reality, a typical medical device is more likely to be disinfected with a cleaning solution that contains chlorides. PPSU comes under chemical attack when in contact with chlorides, causing the material to degrade and lose its structural properties. In this instance, more appropriate materials may include polyether ketone (PEK) and polyether ketone ketone (PEKK). Again, however, they’re both costlier options. So it’s best to thoroughly analyze the material possibilities early in design.
|
Materials analysis may include color analysis and physical (as shown here) and chemical testing. |
The need to implement the best-performing material early on is critical for medical devices, given the regulatory approvals that are often involved. A change in material specs would probably necessitate going back to the beginning of a timely, costly FDA approval process.
Speaking of regulatory approvals, additional analysis such as product life cycle testing, which simulates the use and environmental exposures a product is likely to encounter, may be necessary. Such testing is particularly important to devices that will be used daily, as opposed to a product designed for use only in occasional emergencies (such as an automated external defibrillator).
Variables observed in these tests—which may include tensile, flexural, compressive, impact, temperature, and more—provide feedback that gives a clear picture of material performance in specific application conditions. Any failures should then be recreated through FEA, a computer-based modeling tool. Findings are then used to modify the design and the tool as necessary.
The goal is to make a longer-lasting, more reliable end product. In the medical field, this reliability isn’t just the difference between happy and dissatisfied end users; product failure often has more severe consequences that are important to consider in detail up front.
Enhanced Design for Manufacturability
Many medical devices are high tech and highly cosmetic products. The industrial designers for these products are typically focused on the devices’ ergonomics, functionality, and appearance—and less so on how they are manufactured.
Design for manufacturability, however, should not be discounted. Mold flow, PFMEA (process failure mode and effects analysis) and other techniques are important tools in this effort. Detailed simulations help unveil issues that then inform better process, part and mold designs. These processes do take a little more time on the front end. But by anticipating problems and designing out inefficiencies, such services—which OEMs often don’t have room for on their own leaned work forces—can prove very valuable.
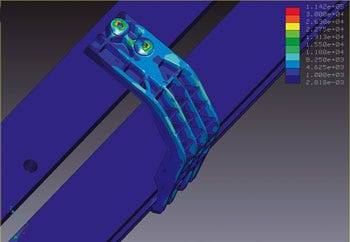
|
Moldflow analysis can help uncover ways to reduce cycle times, eliminate warpage, and create better parts. |
Mold flow, for example, is an excellent tool for examining warping conditions and other problems. The cause may be the gating, venting, ejection or a range of other factors in the tool design. The sophistication of Mold flow models can help pinpoint the cause and lead the way to a tool redesign that counteracts the warp. As a result, overall product development time can be reduced.
The biggest opportunities for improvement lie in simplifying the geometry of the tool and taking out secondary steps. For example, a molder might be able to simplify a tool by eliminating undercuts that add unnecessary costs to a tool. Or, engineers on a molder’s team might figure out a way to core out a part and decrease wall thickness without sacrificing quality—a benefit in both material costs and cycle time.
On the other hand, engineers might be able to identify a way to add into a tool a feature that was originally specified as a secondary manufacturing operation. If designing out secondary operations is not a reasonable solution, design considerations and production planning can take place in advance to eliminate steps from the value stream and allow for the molder to take on more of these ancillary steps. In many cases, printing, decorating, assembly, welding, heat staking, vendor managed inventory, and more can be done all in one place, taking cost out of the final product.
Scientific Molding for Greater Repeatability
Quality and precision are, of course, critical requirements of any medical device. And at any stage of product development, quality is paramount.
|
Stronger, more cost effective products can be designed through working closely with a contract molder from concept through distribution. |
The manufacturing stage is no exception. There is no room for defects. To meet the rigorous standards of medical device design and manufacturing, molding needs to be consistent from shot to shot, cavity to cavity and run to run. A part that is slightly warped or has a bit of flash might be workable in other industries, but it’s unacceptable for a medical device.
Achieving this exact precision isn’t easy and shouldn’t be left to chance, but a methodology called scientific molding can help. Although it’s currently well understood only by about 5% of medical designers and OEMs, scientific molding results is an approach to processing that every medical device maker should get to know.
Here’s why. Scientific molding is a discipline that pays special attention to the consistency and the robustness of the molding process. During molding trials, scientific molding technology helps calibrate the tool and process parameters to optimize the characteristics of the material. During production, in-mold monitoring via state-of-the-art sensors and software ensures part quality checking for consistency in every part. The monitoring focuses on molding pressure in the tool, data which are used to derive optimal fill speed, pack time, and pressure.
The objective is that no matter what technological variables come into play, the process remains consistent and repeatable, removing all guesswork from an incredibly complex discipline. Scientific molding plays a role when the molding process is being developed at the start of molding trials, and it is used for quality control during production.
Faster Speed to Market (and Other Positive Outcomes)
As in any market, timelines are critical in the medical device industry. But as mentioned earlier, an industrial designer is typically focused more on issues such as a product’s functionality and appearance. The understanding that a product slow to market will result in the loss of sales dollars can sometimes be overlooked in the designer’s creative atmosphere.
Project management experience and commitment are integral to moving the production process forward with discipline and all due expediency. A project manager can identify the key milestones where decisions need to be made and designs have to be finalized. The project manager ensures that milestones are met, resulting in the product being delivered to market on time and on budget.
Given today’s accelerated product development time tables, rapid prototyping and other additive fabrication services may also benefit the product and design process. Additive technologies can bring a design into a solid model, and eventually, into a physical part.
Rapid prototyping methods are in various stages of development, but here are a few of the more common examples that some contract molders offer:
Stereolithography (SLA) builds a part layer by layer with a liquid photopolymer according to 3D CAD data.
Selective laser sintering (SLS) is a process in which a powerful laser fuses particles of any of a variety of materials into layers built upon each other to form the completed model.
Fused deposition modeling, which is most useful for small parts with fine detail such as those found in the medical field, also builds parts by layering and allows for material trade-offs between strength and temperature properties, helping it withstand more testing than other prototype options in many cases.
The trend in the technology, however, is to focus on rapid prototype tooling so that prototypes can be molded in their production-intent materials. So much more testing can be done with a prototype part from its production-intent material than one made from the fast-curing resins used in the processes mentioned above. This also speeds up development time because many of the tests performed on the prototype made from the production-intent materials do not have to be repeated on the production part. The part is qualified in the prototype phase.
These techniques can help cut development time and costs, as well as improve overall efficiency of product development by exposing potential problems that might not be as obvious in the data on paper. Models can be built quickly and used for fit and functional testing, marketing presentations, and other needs. One of the biggest advantages is that everyone in the value stream can get a look at a lifelike sample. This way, they are better able to identify opportunities to improve the part design, mold design, assembly, or some other aspect of the end product.
Among the most common problems exposed by a functional prototype are assembly challenges. Parts might look like they assemble on the computer screen, but until the physical parts are in your hand, its impossible to know. Other issues are ergonomics and cosmetics. Things might look good on the screen but until you have felt the part, used the part, or have seen the part in natural sunlight, you don’t really know what you have.
Conclusion
When a molder is brought on after a design is complete and approvals are secured, an OEM misses the opportunities and positive outcomes discussed here. OEMs that work closely with their molding partners, on the other hand, stand to gain a significant competitive edge.
Tom Walczak is general manager of Dickten Masch Plastics’ thermoplastics plant in Nashotah, WI. He can be reached at [email protected].
About the Author(s)
You May Also Like




.png?width=300&auto=webp&quality=80&disable=upscale)
