A Medical Device FDA Clearance for the History Books
Trivia Tuesday: What first-of-a-kind implantable device did FDA clear in 2013?
December 20, 2022

Back in 2013, Oxford Performance Materials made medtech history by scoring the first FDA clearance of a 3D-printed polymer cranial implant.
The OsteoFab Patient Specific Cranial Device (OPSCD) is additively manufactured from PEKK (polyetherketoneketone) polymer. The following year, FDA cleared another first-of-a-kind device made by OPM: The OsteoFab Patient Specific Facial Device, which became the first 3D-printed facial implant to receive FDA clearance.
Today, the South Windsor, CT-based company sells a whole line of OsteoFab implants that are produced using the company's proprietary 3D printing process. The implants can be designed from a patients MRI or CT scans for custom solutions, or using traditional design methods for series production of 3D-printed standard implants. The 3D-printing company says its OsteoFab technology helps reduce intricate assemblies, eliminates manufacturing tooling, and prevents the need for coating or other post-processing surface modification.
While originally cleared in 2013 for two-stage cranioplasty procedures, FDA has since cleared an expanded indication that allows the OPSCD to be used to fit pre-planned, virtual defects in the instance of single stage cranioplasty procedures. With the expanded single stage indication, a virtual defect can now be created and a patient-specific cranial implant can be designed to fit the planned defect that will be created during the operation. Ultimately, the single stage approach for cranioplasties results in less operating room time for the patient and surgeon, lower costs, and a better level of care, according to the 3D-printing company.
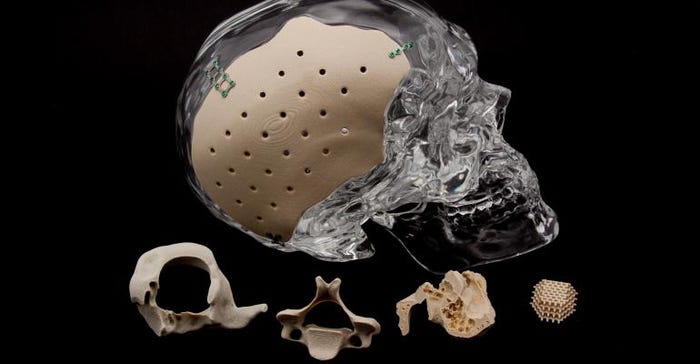
In addition to the OPSCD and the OPSFD, which are both distributed by Zimmer Biomet, the OsteoFab platform includes 3D-printed suture anchors and a 3D-printed vertebral body replacement system.
As with a lot of medical devices, having the right material is key to OPM's success.
“We focus on 3D printing as a process,” Scott DeFelice, the company's founder, chairman, and CEO, told MD+DI in a 2014 interview. “It's nice that you can make shapes and things with a 3D printer, but it doesn't matter if they're not made of the right material.”
OPM's proprietary OXPEKK polymer's powder formulation allows OPM to print implants that are biocompatible and mechanically similar to bone.
“It's osteoconductive and offers a biological approximation, what we call biomimicry, so that bone grows onto and into the implant.” DeFelice said.
Orthopedic implants have seen a lot of advancement with the rise of 3D printing, a trend that is likely to continue in 2023, according to Ryan Martin, CEO at Fathom Digital Manufacturing.
"One area that’s really embraced 3D printing technology is medical orthopedic implants," Martin said in a statement recently provided to MD+DI. "Although there are lots of applications in this field, ranging from hips, knees, or even shoulders, the ability to customize to improve the patient experience is all made possible with additive manufacturing. You can create a structure that’s bone-like with 3D printing, which allows patients to heal much faster because the body responds to these orthopedic implants more quickly.”
Check back next week for a new Trivia Tuesday question on our home page and test your knowledge about the medical device and diagnostics industry!
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
