Must-Know Standards and Tests for Wireless Medical Devices
Knowing which radiofrequency standards and tests to choose can speed time to market and lower the cost of wireless medical devices.
The development of new wireless technologies continues at an ever-rapid pace, primarily driven by the consumer electronics industry. Although it seems logical to apply the same leading-edge technologies to medical devices, doing so without careful forethought of the ramifications could be disastrous. Telehealth-based wireless connectivity is challenged to stay abreast of product evolution while ensuring safety and quality of service.
|
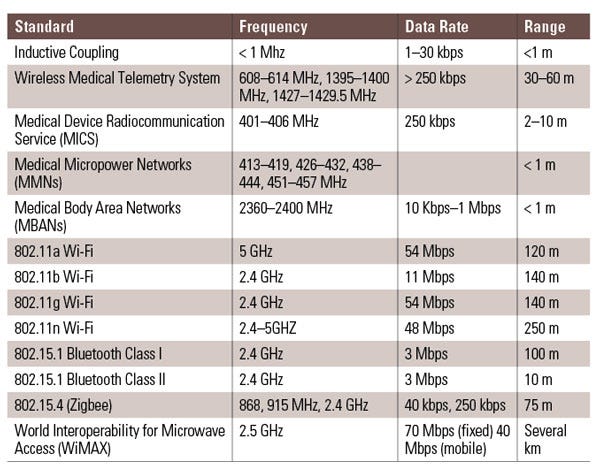
Table 1. Shown are common wireless standards and RF frequencies for wireless medical product design targeted for U.S. medical devices. |
Proper selection of a specific radio-frequency (RF) standard depends on determining the best fit for the product’s intended use (Table 1). However, many medical device design teams adopt standards within the FCC industrial, scientific, and medical (ISM) bands as the basis for new products. Communication modalities in these bands include all variations of 802.11a/b/g/n, in addition to Bluetooth, ZigBee, and radiofrequency identification (RFID).
The reasons to select one of these methods are simple: time to market and availability of ready-made radio integrated circuits (ICs). Using other FCC-defined frequency bands can be challenging for many companies. Only the largest organizations have the financial power to architect radio IC silicon in-house to simplify product designs. For a start-up or smaller company, options for radio IC component selection can be limited or nonexistent.
The FCC continues to add new spectrum for new standards such as the medical micropower networks (MMNs) and medical body area networks (MBANs), but industry adoption may be slow. Though consortiums, such as IEEE 802.15.6, have been working to define a MAC/PHY interface that can serve MBANs, the question remains: Will industry be able to procure readily available silicon? In addition, while MMN, a technology standard to connect microstimulator implants that can use electrical charges to activate or stimulate limbs, is exciting, it suffers the same chasm-crossing adoption challenge of having no off-the-shelf ICs available to support product design success.
Without adoption of new FCC frequency allocations, the safety and quality-of-service delivered by any wireless medical product is questionable. Ensuring wireless communication interoperability and coexistence has become a focused effort for FDA, especially since many wireless medical designs are adopting ISM-based standards. Finding training materials on RF interoperability and coexistence testing to satisfy a risk-based design flow are difficult. The FDA guidance document “Radio-Frequency Wireless Technology in Medical Devices,” outlines concerns related to RF wireless devices and asks that suppliers specifically address risk management, design development considerations, verification testing, validation testing, and labeling for their chosen wireless modality. Test engineers reading this article will find that the guidance lacks specific methods for testing and that one must sort through the plethora of industry materials to find procedures that apply to FDA concerns. The sidebar “Wireless Test Plan Considerations” outlines a limited set of standards to assist in wireless medical device testing, whereas it is still recommended that design teams review coexistence test plans with known experts in the field of product deployment.
Network Coexistence and Interoperability
With this in mind, the existing IEEE 802.15.2 guideline for coexistence should be considered. It defines wireless coexistence as the ability of one wireless system to perform a task in a given shared environment, where other systems in the same environment have an ability to perform their tasks, and may or may not be using the same set of rules. In conjunction with electromagnetic compatibility (EMC) testing, designer risk documentation should include network-level testing. Notably, there is a clear distinction between EMC testing and wireless coexistence testing. FDA advises network coexistence testing should demonstrate that RF wireless devices and networks in close proximity to one another are not dramatically affected by the wireless medical device under test. Also, wireless medical device functions should not pose an unacceptable risk to the user when the device is functioning in an adverse RF environment subject to interaction.
Current methods of wireless coexistence testing use ad hoc methods that vary widely among device manufacturers and test facilities. Moreover, current medical device EMC standards have no requirements or test procedures to assess the performance of systems containing RF receivers in the presence of in-band transmitters. Test engineers should review IEEE 1900.2: Recommended Practice for Interference and Coexistence Analysis. This document includes an extensive list of coexistence factors to consider during testing and recommends interference analysis criteria for measuring interference between radio systems.
Various groups interested in wireless medical device coexistence testing are already at work. An ANSI-accredited subcommittee, C63.27, held preliminary teleconferences and meetings in 2012. Another active group providing research on network coexistence is the University of Oklahoma Wireless and Electromagnetic Compliance and Design Center (WECAD; Tulsa, OK). WECAD has analyzed network coexistence using simulated and real-world network traffic, including a worst-case perspective. Measurements include performance benchmarking, bandwidth utilization, packet latency, and maximum data rates. Also of importance to WECAD publications is inclusion of line-of-site (LOS), and nonline-of-site (NLOS) testing.
Achieving RF coexistence lies in the ability to control one of three factors in the physical layer—frequency, time, and space—as outlined in the following test setup example. Coexistence is possible given adequate frequency separation between wireless networks, sufficient distance between wireless networks, effectively decreasing the signal-to-interference ratio (SIR) in each, or relatively low overall occupancy of the wireless channel. The logical domain above the physical layer is important to interoperability, covering all the behaviors of the higher open systems interconnection (OSI) layers, including media access control (MAC), and routing. Test engineers must remember that the physical layer is not the only thing to consider when providing high-quality communications. A system must combine smart channel selection with higher layer MAC and networking mechanisms.
Wireless Medical Device Testing Setup
While every test plan is unique, a series of steps can be used in the planning process to ensure the right test plan is developed for a product. An example based on radiated wireless coexistence testing, as it is the most complex test setup, follows.
Choose a Test Setup. When defining a test plan for wireless coexistence, the first step is to choose a test setup that is conducive to the medical device. If the wireless medical device does not have an easily accessible antenna port, it will be difficult to perform conducted wireless coexistence testing. It should also be noted that FDA prefers to see the full system under test, including complimentary medical devices in the interfering network’s field during testing that may not be affiliated with the wireless link under test. This limits a two chamber test setup in its applicability.
Identify Potentially Interfering Devices. After a test setup has been chosen, the second step is to identify possible interfering devices operating in the medical device’s frequency band. One way to limit the frequency span of possible interferers is to use EMC radiated immunity test results. If the medical device is unable to communicate wirelessly over a frequency span during EMC immunity testing, then that is one possible frequency span where wireless interference can occur. This is because the electric field generated by an interfering wireless network will probably not be on the order of magnitude of the electrical field generated during immunity testing.
Determine the Method for a Standardized Physical Layer Test. After these two steps are completed, the methodology for a standardized physical layer test is taken. In terms of power, the received signal strength of the wireless nodes under test is suggested to be minimum while still ensuring a 0% packet error rate (PER). The minimum received signal strength (RSS) can be theoretically found based on the wireless standard receiver sensitivity and coding gains. This will give an estimate and then verify the minimum RSS experimentally.
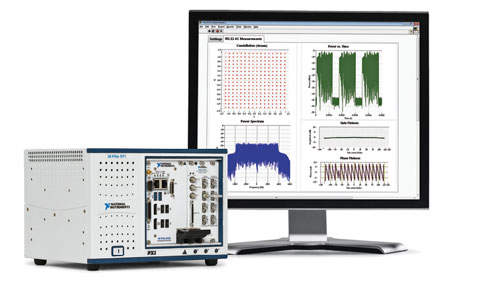
Figure 1. The National Instruments vector signal transceiver combines a vector signal analyzer and vector signal generator with a user-programmable field-programmable gate array for real-time processing and user-defined zsoftware-defined radio standards such as 802.11ac, ZigBee, and RFID. |
Introduce the Interfering Network. After the wireless medical device nodes have been placed, the interfering network is introduced into the test setup. The separation distance, or space, between the wireless node under test and the interfering network is based on ANSI C63.18. The ANSI C63.18 standard is the recommended practice for an on-site, ad hoc test method for estimating radiated electromagnetic immunity of medical devices to specific RF transmitters. Even though not dealing with electromagnetic immunity in coexistence testing, the recommended practice sets guidelines for the initial and minimum separation distance between the nodes based on the transmitting power of the interfering network. During testing, the maximum transmit power is used for the interfering network and the autopower is disabled during testing.
In terms of frequency, if applicable for the two networks, the wireless medical network and the interfering network are set to the same frequency, or cochannel. A test engineer should also test for adjacent channel interference by increasing the separation frequency between devices. However, in some cases, cochannel interference may not be possible when testing with Bluetooth. In this case, the interfering network, such as Wi-Fi, should be set to the middle channel of the Bluetooth network, which maximizes adjacent channel interference.
In terms of time, set the duty cycle of the interfering network to its maximum, and if interference is persistent, decrease the duty cycle of the interfering network. Figure 1 presents a test equipment setup using the recently launched National Instruments vector signal transceiver, which is a PXI-based software-designed instrument. FDA prefers that the interfering wireless network be an actual network, such as an 802.11 access point and client. However, with advances in software-defined radios (SDRs), it is now possible to accurately characterize and emulate a wireless network to have the same power, frequency, modulation, packet size, variable duty cycle, and impairments exhibited in an actual network. Such capabilities expand test scenarios beyond traditional methods to advance product quality while also helping to lower the cost of testing.
Conclusion
As noted, proper selection of a specific RF standard for a new medical device depends on determining the best fit for the product’s intended use. The FCC continues to add new spectrum for new standards such as MMNs and MBANs, but industry adoption may be slow until off-the-shelf radio IC become available. No matter the radio technology selected, it is the product developer’s responsibility to use a risk-based approach and test for RF coexistence. Standards and methods outline in this article assist in building a complete test strategy to ensure product operation and saftly. Wireless technologies will continue to advance telehealth-based solutions, enabling physicians to interact with patients who have geographic or socioeconomic limitations. These advancements are translating into observable outcomes such as increased patient satisfaction with care, improved clinical outcomes, and cost savings.
Greg Crouch serves in the life science segment at National Instruments (Austin, TX). He has worked at the company for 24 years, serving in R&D, marketing and sales management roles.
Nick LaSorte earned a PhD at the University of Oklahoma, Tulsa and was an FDA Research Fellow. He continues to research medical device RF coexistence while participating in standards groups.
Related Content
Wireless Test Plan Considerations
MBANs Could Advance Patient Care, But Interoperability Is a Concern
About the Author(s)
You May Also Like