Should you track adverse events differently?
March 23, 2017
A new solution allows users to track compliance pertaining to events and corrective actions using either mobile devices or via Web browsers.
 Managing corrective actions is one of medtech companies' most complex tasks. Many companies, however, continue to use simple solutions like spreadsheets, observed Joseph Randazzo, Vice President of Strategic Growth for EtQ.
Managing corrective actions is one of medtech companies' most complex tasks. Many companies, however, continue to use simple solutions like spreadsheets, observed Joseph Randazzo, Vice President of Strategic Growth for EtQ.
"About 3 years ago we noticed that not a lot of medium-sized companies were being served by a typical quality suite," Randazzo told Qmed. Many were using email, spreadsheets, notes, reminders, and to-do lists, all of which could be lost or overlooked and provide no path for trending and improvement, he reported.
So EtQ surveyed companies to understand user needs. "Users were looking for out-of-the box solutions at a lower-price point," said Randazzo. "And they wanted one place to store documentation on all their events."
As a potential solution, EtQ took its successful namesake platform for quality management and simplified it so users could get it up and running in just hours. Verse Solutions was born. But Randazzo said some companies still stuck with their spreadsheets.
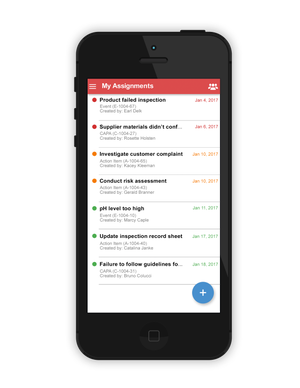
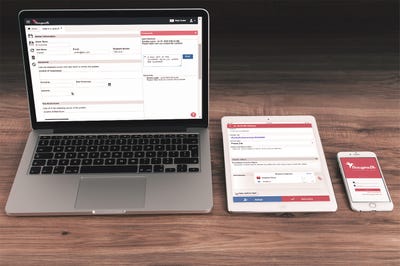
The company then decided to do something pretty bold--offer free software through an application--so it built Traqpath. The event-tracking solution allows users to conduct compliance tracking activities for events and corrective actions using either mobile devices or via Web browsers. The app can be downloaded from the Web, the App Store, or Google Play and used immediately.
"With Traqpath, users can track events, including adverse events, and then manage corrective actions and track compliance," Randazzo said. Anything that happens regarding a nonconformance can be considered an "event," such as a quality issue, an inspection, a customer concern, an adverse event, or anything that has an impact on the quality, regulatory, or compliance of the organization, he reports. Serious, systemic, or pervasive events get escalated to a corrective action. Traqpath guides users--both mobile and Web--through these steps.
"It is broad enough to encompass any event--really anything the user wants to track," said Randazzo.
The system allows users to involve all stakeholders, both internal and external. "There's an external assignment feature allowing users to email requests to suppliers," he says. Up to 10 workgroup members can use Traqpath Free along with unlimited external stakeholders.
Built specifically for life sciences companies, Traqpath accommodates electronic signatures and records, as required by FDA 21 CFR Part 11, as well as a full field and record history.
Randazzo describes one use case involving a medical device manufacturer that was dealing with several mergers and acquisitions. The parent company noticed that many of the acquired companies' quality systems "weren't up to snuff," recounted Randazzo. They needed efficiency and a common language, but not necessarily access to the parent company's operations, he explained. Traqpath provided a simplified place for tracking and documenting compliance.
Randazzo says that for companies with evolving needs, Traqpath offers a scalable approach--users can transition from the free to the standard solution.
EtQ will be exhibiting at Booth #474 at Medical Design & Manufacturing (MD&M) East June 13-15 in New York City.
Daphne Allen is executive editor of Pharmaceutical & Medical Packaging News and a contributor to Qmed. Reach her at [email protected] and on Twitter at @daphneallen
About the Author(s)
You May Also Like




