Philips Scores FDA Clearance for Informatics Platform
July 5, 2017
Clearance for the IntelliSpace Portal 9.0 opens the door for a range of new applications for radiology, neurology, oncology, and cardiology.
Kristopher Sturgis
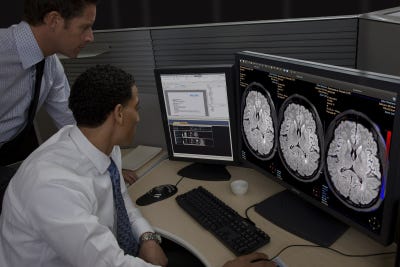
The newest FDA-cleared application for Philips' Intellispace Portal is the Longitudinal Brain Imaging (LoBI).
FDA has cleared a new comprehensive visualization platform from Philips that is designed to offer a single, integrated solution in radiology, neurology, oncology, and cardiology for advanced analytics and diagnosis. The IntelliSpace Portal 9.0 can be used to help streamline care by giving clinicians a comprehensive overview of each patient, the company said.
The technology has been available outside the U.S. since November of last year. Recent innovations to the platform have added more than 70 new applications to the portal, all of which can be used to help better quantify data and diagnose conditions using multimodality clinical applications that have been optimized for patient evaluation over extended periods of time.
The platform has been in use with a few different development partners while the company went through FDA's 510(k) clearance process, and the performance has been largely successful. Mark van Buchem, a professor of neuroradiology at the Leiden University Medical Center where the technology was piloted, said the platform helps clinicians visualize and quantify the most subtle manifestations of disease and differences over time, adding significantly to the diagnostic process and overall patient care.
The latest clinical advance on the platform cleared for the U.S. market is the technology's Longitudinal Brain Imaging (LoBI) application. The LoBI app was designed to analyze brain images to support the evaluation and diagnostic processes of neurological disorders that develop over time. The application can now help clinicians monitor the progression of a wide range of neurodegenerative disorders from stroke and Alzheimer's disease to multiple sclerosis.
The platform is even equipped with a multimodality tumor tracking capability that offers a new innovative method for enhanced tumor volume measurements based on MRI and CT scans. This new function aims to improve upon the current standards for cancer treatment follow-ups by providing a visual indication of how tumors are progressing, and how the cells are responding to treatment.
The company expects to continue to evolve the platform to enhance its capabilities and expand its functions. Last week Philips said it unveiled plans to acquire Electrical Geodesics for $36.7 million, a move that could potentially bolster the continued development of the platform. Electrical Geodesics' portfolio of hardware, software, and acquisition sensors aim to complement Philips' existing imaging technologies and possibly improve the advanced informatics portfolio for neurological applications.
Kristopher Sturgis is a contributor to Qmed.
[Imaging Credit: Royal Philips]
About the Author(s)
You May Also Like


