Fresenius Kabi USA Infusion Pump Software Recall Declared Class I
The company is urging customers to update the device software in order to mitigate noted software anomalies.
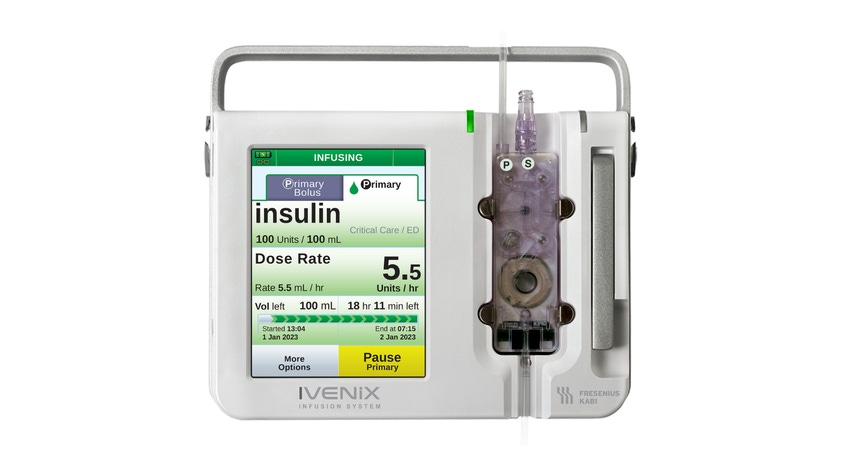
Fresenius Kabi USA recently announced the recall of its Ivenix Infusion Pump LVP software has been identified as Class I by FDA. The Ivenix Infusion system is indicated for both hospital and outpatient care use. It is used for the controlled administration of fluids to patients through methods such as IVs, arterial lines, epidurals, and subcutaneous delivery. The fluid administered include medications, blood products such as red cells or plasma, or other fluids need for treatment of adults and children, as well as neonates and infants.
The recall, which is a correction, not a product removal, is not focused on the system itself, but instead on the device’s infusion pump software. Fresenius Kabi said the reason for the recall was multiple noted software anomalies that were occurring which had the potential to cause serious patient harm of death.
In response, the company is releasing a new software version (5.9.1) to remedy the issue.
On March 7, Fresenius Kabi sent an Urgent Medical Device Field Correction to its customers requesting all to update LVP’s to software version 5.9.1 by reaching out to a company representative to have it installed.
There have been no reports of injuries or deaths associated with the recall.
“Fresenius Kabi initiated a software update in early March 2024 for its Ivenix LVPs that included both software enhancements and additional safety features to bring its pumps to the newest software status,” a spokesperson from Fresenius Kabi told MD+DI. “It is important to note there have been no reports of patient harm received from Ivenix pump customers related to this software update. Fresenius Kabi informed the FDA of this need for a software update and following FDA’s review, it classified this as a recall in mid-April and published a notification on April 17, 2024. These software updates were done remotely, so no pumps were returned to Fresenius Kabi or taken out of use. Last month we informed all affected customers on the required remote software update and the majority (approximately 90%) of customers have implemented the software update accordingly. We continue to contact the remaining customers to have their respective software updates completed as soon as possible.”
About the Author(s)
You May Also Like



