Did Outset Medical Put the TabloCart Before the Horse?
FDA warns the dialysis company that the TabloCart, which Outset launched last year, requires FDA clearance.
July 11, 2023

Dialysis tech company Outset Medical appears to have put the cart before the horse, so to speak. The "cart" in this case being quite literal – the TabloCart – and the "horse" being metaphorical for FDA clearance.
The San Jose, CA-based company develops the Tablo hemodialysis system, which has been compared to the Keurig coffee maker in that it offers a single-serving, dialysis-on-demand approach. And, unlike conventional dialysis machines that are hooked up to a bulky water-processing facility, the Tablo system requires only an electrical outlet and tap water. The system is designed to make the patient’s prescription dialysate in real time, during the treatment.
"It's sort of the K-Cup of dialysis," CEO Leslie Trigg told MD+DI in a 2017 interview.
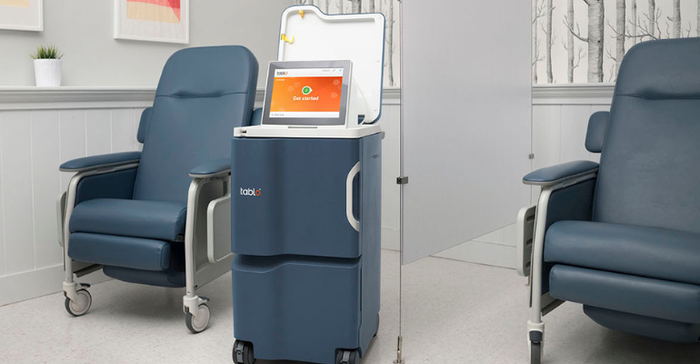
Recognizing the need for enhanced maneuverability as well as an optional prefiltration method, the company rolled out the TabloCart last year that is sold as an accessory to the Tablo system. The TabloCart provides added maneuverability and the option of either additional filtration configurations (depending on a facility’s incoming water quality), or storage. The prefiltration option includes a water pressure booster pump, and a visual display for filter life and cart servicing.
Now, Outset Medical is facing an FDA warning letter because the TabloCart requires a 510(k) clearance. The warning letter also raises an issue with the company promoting its hemodialysis system for continuous renal replacement therapy, a modality outside of the current indications for the Tablo.
Outset said in a filing with the U.S. Securities and Exchange Commission that sales of the TabloCart have not been material to the company’s financial results. The company also said it intends to work collaboratively with FDA to resolve the issue, including potentially submitting for a 510(k) clearance for the TabloCart.
According to the company’s SEC filing the warning letter does not ask Outset to restrict the manufacture, production, or shipment of the Tablo nor does the agency want the company to take Tablo off the market.
Outset said it will respond to the warning letter within 15 business days, but there is no guarantee that FDA will be satisfied with the company’s response. There are also assurances as to how quickly the matters raised in the warning letter can be resolved.
The warning letter follows an earlier Form 483 in which FDA identified four observations resulting from an FDA inspection that concluded on February 10. These observations related to a single medical device report that was not submitted within the required time; completeness of software validation documents; procedures defining escalation of non-conforming product to corrective and preventive action (CAPA); and procedures connecting actions in customer complaints and the CAPA system. Outset said it provided its response plan for those observations on March 3 and has since completed the associated remediation workstreams to fully address those observations.
Outset’s shipment halt
In June 2022, Outset had to place a shipment hold on its Tablo for home use because FDA had not yet completed its review for changes made since the system’s original March 2020 clearance. The company secured that FDA clearance and resumed shipment for home use in August. By that time, however, Outset had already lost momentum on the home front as patients chose other dialysis solutions.
“And as time has marched on here, it became apparent that the uncertainty around FDA timing led those patients to choose other paths, which is completely understandable, but now that those cards have been turned over, we now know that we need to build a new patient pipeline largely again from scratch,” Trigg said during a call with investors last August, according to Seeking Alpha transcripts.
About the Author(s)
You May Also Like



